INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic functional bowel disorder (FBD) characterized by episodic abdominal pain and altered bowel habits that affects ∼12% of the global population [Reference Halvorson, Schlett and Riddle1–Reference Barbara3]. The aetiology, pathogenesis and prognosis of IBS are not well understood and there is no widely effective treatment [Reference Baber4–Reference DuPont9]. IBS causes significant morbidity, places substantial burden on healthcare systems and can greatly affect quality of life [Reference Haagsma10–Reference Garcia Rodriguez and Ruigomez17]. In the USA, IBS accounts for 2·4–3·5 million physician visits annually, costing an estimated US$30 billion [Reference Longstreth13]. In The Netherlands, the annual disease burden of post-infectious (PI)-IBS associated with Campylobacter spp. and Salmonella spp. was recently estimated to be 2300 disability-adjusted life years (DALYs), potentially accounting for nearly 50% of the total burden for these pathogens [Reference Haagsma10].
There is increasing evidence that acute infectious gastroenteritis (GE) can increase the risk of IBS, with several studies demonstrating that a significant number of GE patients develop PI-IBS [Reference Halvorson, Schlett and Riddle1, Reference Thabane, Kottachchi and Marshall2, Reference Parry18–Reference Mearin23]. Campylobacter, Shigella, Salmonella and Escherichia coli infections as well as viral agents, including norovirus, and parasites have been associated with PI-IBS [Reference Borgaonkar5, Reference Marshall21, Reference Mearin23–Reference Gradel25]. It has been hypothesized that exposure to an infectious organism alters gut flora, increases intestinal permeability and triggers chronic inflammation, inducing PI-IBS [Reference Barbara3, Reference DuPont9, Reference Dunlop, Jenkins and Spiller26, Reference Marshall27].
Studies of PI-IBS incidence and risk factors have yielded varied results and few, if any, have identified unique causal infectious agent(s) [Reference Halvorson, Schlett and Riddle1, Reference Thabane, Kottachchi and Marshall2]. Severity of GE (duration of diarrhoea, presence of blood in stools, abdominal cramps, weight loss), younger age and female gender have been associated with increased risk of PI-IBS [Reference Marshall21, Reference Gwee28–Reference Thabane34]. Anxiety, depression and major life events have also been identified as potential risk factors with some hypothesizing that these disorders prolong gut inflammation and heighten sensory perception [Reference Ruigomez, Garcia Rodriguez and Panes22, Reference Dunlop, Jenkins and Spiller26, Reference Gwee28, Reference Neal, Hebden and Spiller29, Reference Moss-Morris and Spence32, Reference Gwee35–Reference Tobin37]. Several studies have found increased healthcare-seeking behaviors in IBS patients but reasons for this are not well understood [Reference Hyams15, Reference Pare16, Reference Nicholl33, Reference Spence and Moss-Morris38]. Antibiotic use during or after GE has also been associated with increased risk of IBS [Reference Ruigomez, Garcia Rodriguez and Panes22].
Understanding the epidemiology of PI-IBS will contribute to a better understanding of the public health impact of the disease and potential preventive strategies. The primary purpose of this prospective cohort study is to estimate the relative risk (RR) of IBS following GE and explore potential risk factors in the Dutch primary-care population.
METHODS
A prospective cohort study was undertaken using electronic medical records from the Primary Care Network Utrecht (PCNU), a collaboration between Julius Centre of Utrecht University Medical Centre and 38 general practitioners (GPs) from six Utrecht primary-care centres. On average, 60 000 patients are seen annually with each patient being registered with a single practitioner. Routine healthcare data, including diagnosis, prescriptions and referrals, are recorded in a centralized database for all primary-care consultations. Diagnoses are coded using the 2nd edition of the International Classification of Primary Care (ICPC) from Wonca International Classification Committee (WICC). Prescribed medications are recorded using the World Health Organization's Anatomical Therapeutic Chemical (ATC) classifications.
The sample population was all PCNU patients, aged 18–70 years, seen between 1998 and 2009 with at least 1 year's electronic data. Patients with pre-existing diagnoses of cancer, alcohol abuse, inflammatory bowel disease, IBS, FBD or abdominal surgery, or ⩾5 prescriptions associated with IBS or FBD treatment were excluded from the analysis. Since GE symptoms may actually be undiagnosed IBS symptoms, patients with GE symptoms in the previous 12 months were also excluded.
Patients clinically diagnosed by their GP with GE during the observation period were identified as the index group (GE patients). Subsequent GE consultations within 30 days of the initial consultation were considered part of the same illness episode. To assess sensitivity, multiple definitions of GE were considered (Table 1) but only GE clinical results are presented. Patients were considered to have met a specific definition of GE if they had an exposure meeting that definition within 30 days of their original, matched exposure. Randomly selected PCNU patients consulting for non-GE symptoms, matched by age, sex and time of visit (within 1 month), served as the reference group (non-GE patients). Patients who developed IBS within the first 3 months were considered to be undiagnosed cases at study entry and were excluded.
Table 1. ICPC codes selected for gastroenteritis (GE) and irritable bowel syndrome (IBS)

* Patients may have had multiple different ICPC codes so numbers are not additive. For IBS, number of events presented are during entire follow-up period (1998–2010).
The primary outcome of interest was IBS (Table 1). An incident case of IBS was defined to be a new diagnosis of IBS following GE exposure for GE patients and matched exposure for non-GE patients.
For GE patients, potential risk factors and comorbidities were evaluated: age at onset of GE, gender, socioeconomic status, bloody stools, abdominal cramps, weight loss, psychosocial factors, high consultation frequency and high prevalence of unexplained symptoms. Subjects were considered to have bloody stools, cramps or weight loss if they had an ICPC diagnostic code for these events within 7 days of the GE event. Consultations in the year prior to study entry with psychological (P category) or social problems (Z category) ICPC codes were used to identify psychosocial factors. Since consultations for fear or unexplained symptoms may indicate underlying psychosocial problems, ICPC diagnostic codes related to these events were also included (see online Supplementary Table S1).
It was determined that a sample size of 4206 per cohort group would provide at least 90% power, at a significance level of 0·05, to detect a RR for IBS of 2, which is a 100% increase.
The unadjusted RR of developing IBS after GE was estimated at 6 months, 9 months, 1 year, 2 years, 3 years, 4 years, 5 years and at any time point. The RR at 1 year was also estimated using negative binomial regression to adjust for age, gender, time of visit, and practice. For sparse data with zero cells, the RR and 95% confidence intervals (CIs) were estimated by adding 0·5 to each cell and using standard equations. Risk factors and comorbidities for IBS in GE patients were tested using univariate and multivariate logistic regression (backwards elimination). Due to the large number of comparisons and the potential for spurious results, risk factors and comorbidities were only considered significant if 95% CIs from both the univariate and multivariate analyses did not contain 1 and the P value from the multivariate analysis was < 0·02. Survival analysis techniques were used to analyse time to IBS diagnosis and confirm risk factors. The impact of medical registration system was assessed by repeating the analysis separately for each system (not shown). Statistical analyses were performed using SAS v. 9.2 for Windows (SAS Institute Inc., USA).
RESULTS
Sample characteristics
Between 1998 and 2009, there were 728 937 patient-years of follow-up in the PCNU database and 2464 patients diagnosed with GE. A matched sample of 2462 patients consulting with non-GE symptoms was drawn for the reference group (Fig. 1).

Fig. 1. Summary of patients with gastroenteritis (GE) exposure during the study period.
Demographic characteristics, healthcare-seeking behaviours and comorbidities are presented in the online Supplementary Tables. About half of GE patients were female with an average age of 37·85 years. The distribution of socioeconomic status was similar across groups (P = 0·9901). GE and non-GE patients visited their primary-care physician a mean of 6·2 and 2·39 times per year, respectively, throughout the study period (P < 0·0001). Only 9% of non-GE patients consulted ⩾5 times annually during the study period compared to 39% of GE patients (P < 0·0001).
Most (84·6%) GE patients had only one exposure to GE during the study period. Similar proportions of GE and non-GE patients had concomitant diagnoses of bloody stools (0·4% vs. 0·0%, P = 0·0114), weight loss (0·0% vs. 0·0%, P = 0·9995) and history of ulcer (0·1% vs. 0·0%, P = 0·5641). Higher proportions of GE patients had concomitant abdominal cramps (1·4% vs. 0·1%, P < 0·0001) and symptoms of dyspepsia (3·4% vs. 0·8%, P < 0·0001) compared to non-GE patients. GE patients were also more likely than non-GE patients to have a history of psychological problems (10·3% vs. 5·2%, P < 0·0001), social problems (3·3% vs. 2·0%, P < 0·0001), fear of disease (3·1% vs. 2·6%, P = 0·2688) and unexplained symptoms (5·0% vs. 3·5%, P = 0·0109).
Outcomes of interest
Between 1998 and 2010, there were 2358 consultations in the PCNU for IBS. Over half of IBS cases were between the ages of 18 and 40 years. Most (75%) were female. Four PCNU practices reported similar consultation rates for IBS (range 18–22%) while the remaining two practices reported much lower consultation rates (range 9–12%). Over half of IBS cases seen in the PCNU during the study period had a mean consultation frequency of more than four consultations per year.
Of the 2464 GE patients, 36 developed IBS within 3 months of their study exposure and, along with their reference patients, were excluded from the analysis. Of the 2462 matched non-GE patients, 72 were excluded due to prior history of IBS (n = 67), prior history of GE (n = 2) and developing IBS within 3 months of study entry (n = 3). Therefore, 2428 GE patients and 2354 non-GE patients were included in the analysis.
During the study period, 4·9% of included GE patients were diagnosed with IBS compared to 0·9% of non-GE patients. At 1 year post-exposure, the incidence of IBS in GE patients was 1·2/1000 person-years compared to 0·3 in non-GE patients. At 1 year, the adjusted RR of IBS following GE was 4·85 (95% CI 2·02–11·63) (Table 2). At 5 years post-exposure, the cumulative adjusted RR of IBS was still significantly higher in GE patients than in non-GE patients (RR 5·40, 95% CI 2·60–11·24) (Fig. 2). With the exception of confirmed GE cases, this increased risk was consistent across GE definitions and time points (see online Supplementary Table S4). For confirmed GE cases (N = 137), there was an increased risk at any time point in the study but not at individual time points.

Fig. 2. Relative risk of irritable bowel syndrome following gastroenteritis. Bars indicate upper (UCL) and lower (LCL) 95% confidence intervals.
Table 2. Relative risk (RR) and 95% confidence interval (CI) of irritable bowel syndrome at 12 months
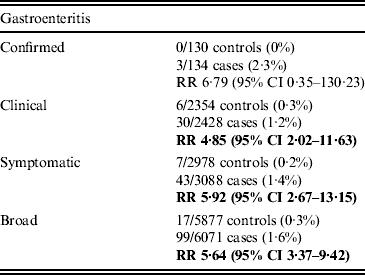
Significant results appear in bold.
Time to event
The median time to IBS was lower in GE patients (555·5 days) than in non-GE patients (863·5). Differences were also seen in the minimum time to IBS (99 days vs. 144 days). The maximum time to IBS was similar between GE (3367 days) and non-GE (3609 days) patients.
Risk factors
Odds ratios, 95% CIs and P values for significant risk factors 1 year post-GE are presented in Table 3. In the univariate analysis, consultation frequency, multiple GE exposures, concomitant cramps, dyspepsia history, psychosocial history and history of fear consultations before study entry were significant. In the multivariate analysis, concomitant cramps and psycho-social history were significant. At any time point, visit year, practice, consultation frequency and concomitant cramps were found to be significant risk factors for IBS in both the univariate and multivariate analyses (results not shown). In the univariate survival analysis, GE exposure, female gender, socioeconomic status, age group, consultation frequency, concomitant cramps, weight loss, dyspepsia history, psycho-social history, social consultations and multiple GE exposures were significant risk factors (Fig. 3 a–f). In the multivariate analysis, female gender, age group, consultation frequency, concomitant cramps, weight loss and psycho-social history were significant risk factors.

Fig. 3. Survival function of irritable bowel syndrome following gastroenteritis by (a) exposure status, (b) number of exposures, (c) consultation frequency, (d) concomitant abdominal cramps, (e) concomitant bloody diarrhoea, (f) history of dyspepsia.
Table 3. Risk factors for IBS at 12 months

OR, Odds ratio; CI, confidence interval; n.s., risk factor was not significant and not included in the final model.
Significant results (ORs and 95% CIs that do not contain 1 and P values <0·02) appear in bold.
DISCUSSION
Foodborne illness is a significant public health issue that can cause significant morbidity and mortality. Several studies have found an increased risk of IBS following acute episodes of foodborne disease but few have examined the risk of IBS prospectively. In the present study using primary-care electronic medical records over a 12-year observational period, we found a fivefold increased risk of IBS after a gastrointestinal infection.
A 2006 meta-analysis of six cohort studies found the median prevalence of IBS in GE patients to be 9·8% (95% CI 4·0–13·3) [Reference Halvorson, Schlett and Riddle1]. A subsequent meta-analysis of 18 cohort studies found a 10% (95% CI 9·4–85·6) pooled incidence of IBS following GE [Reference Thabane, Kottachchi and Marshall2]. A UK study using a general practice research database found the incidence of IBS after GE to be 10/1000 person-years [Reference Ruigomez, Garcia Rodriguez and Panes22]. The incidence in the present study is significantly lower than either meta-analysis or the UK study, which may be a function of the PCNU database. The lower incidence of IBS may also reflect the passive design of a study that employs an existing electronic database of routine care registration data.
We found an increased risk of IBS at 1 year following GE. In our study, GE patients were 4·85 (95% CI 2·02–11·63) times more likely to develop IBS than the reference group. In the UK study, which used Oxford Medical Indexing System and Read diagnostic codes, the RR of IBS following bacterial GE was 2·2 (95% CI 1·5–2·9) [Reference Ruigomez, Garcia Rodriguez and Panes22]. However, an earlier study using the same database found the adjusted RR of IBS to be 11·9 (95% CI 6·7–21·0) [Reference Parry18]. Recent meta-analyses of prospective studies found pooled odds ratios of 7·3 (95% CI 4·7–11·1) and 6·37 (95% CI 2·63–15·40), respectively, for IBS at 1 year. These estimates are higher than our findings which may be due to differences in exposure and outcome definitions. In the present study, we defined incident cases of IBS to be those with an ICPC diagnostic code for IBS and no prior history of IBS. Many other studies have used a stricter outcome definition, requiring multiple visits for IBS in conjunction with medical treatment. Due to our less stringent definition, it is possible that some patients were misclassified as IBS in this study when they may not have met more traditional definitions. However, since the CIs in the present study overlap with those from the meta-analyses, it is not possible to determine if the point estimates are significantly different.
Several studies have examined risk factors for PI-IBS [Reference Marshall21, Reference Gwee28–Reference Thabane34]. As found previously, risk of IBS was higher in females and younger age groups, although this was not significant. Individuals with higher levels of socioeconomic status were less likely to develop IBS at 1 year. The demographic characteristics of the PCNU practices may have contributed to these findings. Many PCNU practices are located in newly developed areas, which could skew the patient population towards younger families with higher socioeconomic status. However, this does not fully account for the increased risk.
It has been hypothesized that GE exposure may trigger an inflammatory response and contribute to hypersensitivity [Reference Wang, Fang and Pan8, Reference Dunlop, Jenkins and Spiller26]. More severe and/or repeated exposures may increase or prolong inflammatory response and are considered to be potential risk factors for IBS [Reference Wang, Fang and Pan8, Reference Gwee28, Reference Ji30, Reference Marshall31, Reference Thabane34]. Our finding of increased risk of IBS in GE patients with concomitant abdominal cramps is consistent with other studies [Reference Marshall31]. Interestingly, bloody diarrhoea, a marker of severe disease, was not a significant risk factor. Weight loss was also not found to be significant, although the guidelines for coding of weight loss in the PCNU are unclear and, as a consequence, weight loss may only be reported in severe cases. As in previous studies, we found an increased risk of IBS in GE patients with a history of dyspepsia [Reference Ruigomez, Garcia Rodriguez and Panes22]. We also found an increased risk of IBS in GE patients with multiple GE exposures, although it is possible that these were undiagnosed cases of IBS. Overall, these findings support the hypothesis that severity of exposure and/or prolonged exposure increases risk of IBS.
Previous studies have hypothesized that psychological stress may increase or sustain inflammatory response to infection and increase the risk of IBS [Reference Ruigomez, Garcia Rodriguez and Panes22, Reference Dunlop, Jenkins and Spiller26, Reference Gwee28, Reference Moss-Morris and Spence32, Reference Gwee35–Reference Tobin37]. To evaluate this, we used a prior history of consultation (within 1 year) for psychological and social problems as a proxy indicator for presence of psychological stress. History of psycho-social consultations was a significant predictor for IBS at 12 months.
Healthcare-seeking behaviours have also been associated with increased risk [Reference Hyams15, Reference Pare16, Reference Nicholl33, Reference Spence and Moss-Morris38]. The hypothesis is that some patients seek help for even minor symptoms and, since IBS is often a diagnosis of elimination rather than confirmation, are more likely to be diagnosed. We used several surrogate markers, including consultation frequency, history of unexplained symptoms and history of fear of disease, to evaluate healthcare-seeking behaviors. Consultation frequency was generally higher in GE patients and was a significant risk factor in the univariate analysis. Since we examined consultation frequency across the study period, it is not possible to determine if consultation frequency was consistent over time or changed with exposure. History of fear of disease consultations was also a significant risk factor in the univariate logistic regression analysis. This may indicate a pattern of seeking help for even minor symptoms or an underlying psychological issue. However, it could also suggest a medical condition with no structural cause, such as IBS. The nature of the association of consultation frequency and history of fear of disease with increased risk of IBS should be explored in a larger prospective study.
Although the primary endpoint was 1 year, we also examined the risk of IBS at 6 months, 9 months, 1 year, 2 years, 3 years, 4 years and 5 years. The hazard function associated with IBS for GE and non-GE patients was also estimated using survival analysis. We found that GE patients had a higher risk of IBS than non-GE patients up to 5 years post-exposure. These findings are curious, as we anticipated an increased risk up to 2 years post-GE that would then level off and trend towards the prevalence of sporadic IBS. The increased risk in GE patients up to 5 years post-GE could be the result of uncontrolled confounders or diagnostic delays. First, patients with GE may suffer from an underlying susceptibility to both GE and IBS. A prospective cohort study of GE patients in Canada found that single nucleotide polymorphisms in genes that encode proteins involved in epithelial cell barrier function and innate immune response were significant risk factors for developing PI-IBS [Reference Villani39]. A Dutch case-control study found that host genetic factors played a role in susceptibility to reactive arthritis and recurrent GE episodes after severe Salmonella and Campylobacter infections [Reference Doorduyn40]. Due to a lack of genetic samples, we were unable to explore the role of host genetics in susceptibility. Second, GE exposure may have changed intestinal microflora, putting exposed patients at increased risk of IBS and GE over time. Finally, physicians may be reluctant to diagnose IBS and may only do so if problems persist over time. Therefore, patients in our study may have had a delayed diagnosis of IBS. The increased risk at 5 years could have also been the result of multiple GE exposures or pathoaetiology of the GE infection. However, since many GE exposures were not laboratory confirmed, it is not possible to determine if this influenced the results. Additional research is needed to understand factors that influence long-term risk of IBS in patients exposed to GE.
There are several study limitations. First, GE patients may not be representative of all patients with GE since many do not seek medical care. Second, healthcare-seeking behaviours may have influenced the results. Since GE consultation may be related to severity of illness, the incidence of GE may be underestimated and the risk of IBS may be overestimated compared to a general population cohort [Reference Spence and Moss-Morris38]. In addition, patients with undiagnosed IBS may seek healthcare more often than non-IBS patients and, therefore, may be more likely to be diagnosed with GE. This would overestimate the risk of IBS following GE. Third, there is potential for misclassification biases. The GE definitions used in the present study include potential IBS symptoms and patients with GE exposure may actually have undiagnosed IBS. Further, there is substantial overlap between upper and lower GI symptoms, such as dyspepsia and IBS, which may be reflected in the diagnostic codes. To address these potential misclassification biases, we evaluated multiple GE definitions and excluded patients with GE symptoms in the previous 12 months and patients diagnosed with IBS within 3 months of study entry. Increased risk for IBS was found across GE definitions and time points, suggesting that these results are not affected by GE definition. Criteria used to diagnose IBS can lead to different classifications of patients [Reference Halvorson, Schlett and Riddle1, Reference Baber4, Reference Boyce, Koloski and Talley6]. However, due to a lack of standards and documentation regarding IBS diagnosis in the PCNU, we could not assess the impact of diagnostic criteria on the study results. Further, in 2006, when three PCNU practices switched to a registration system that required a diagnostic code, the incidence of both GE and IBS increased significantly, suggesting that GPs may be reluctant to formally diagnose GE and IBS otherwise. Stratified analyses were undertaken to further assess the impact of the registration system. The majority of patients were registered before 2006 using a single registration system. Post-2006, a small number of patients (<500 per group) were registered under two new systems, resulting in insufficient power to detect a difference. The impact of registration system on the incidence of diagnostic codes for GE and IBS do, however, raise interesting implications for the use of electronic medical records to evaluate outcomes and should be explored. A lack of data on pre-morbid bowel habits prevented us from identifying patients with undiagnosed IBS. Stool studies to verify the cause of GE in cases and absence of gastrointestinal pathogens in controls were unavailable. Fourth, limited electronic information also precluded inclusion of risk factors found to be significant in previous studies. For example, duration of diarrhoea and smoking have been previously associated with increased risk of PI-IBS [Reference Wang, Fang and Pan8, Reference Gwee28, Reference Neal, Hebden and Spiller29, Reference Marshall31, Reference Thabane34]; however, this information was not recorded in the PCNU database and could not be considered in this study. Finally, we were unable to assess the impact of disruptions in the intestinal microflora, comorbidities and genetic susceptibility on the results.
CONCLUSION
IBS is a significant public health issue that is associated with GE. Patients were at increased risk of IBS 1 year post-exposure compared to unexposed patients and remained so up to 5 years post-exposure, suggesting that not only GE but also other factors may play a role. Concomitant cramps and psycho-social consultations were found to be significant risk factors. Prospective studies are needed to assess the impact of these factors on the development of IBS following GE. Additional research is also needed to understand the biological basis of IBS following GE and clarify causality.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268813001891.
ACKNOWLEDGEMENTS
The authors thank Nicole Boekema at the Julius Centre for her contributions in extracting the data used in this analysis from the PCNU database. Dr Kowalcyk thanks Dr Craig Hedberg and Dr Stephen Kralovic for their input on the development of this paper. This work was funded, in part, by the Food and Drug Administration (Grant 1R18FD004011-01 to B.K.K.).
DECLARATION OF INTEREST
None.










