In recent years, research efforts have been focused on finding alternative protein sources for use in aquafeeds as fishmeal availability has stagnated and market prices have increased( Reference Naylor, Hardy and Bureau 1 ). As a result, compound feeds used in aquaculture today contain increasing amounts of plant protein, replacing fishmeal( Reference Tacon 2 , Reference Gatlin, Barrows and Brown 3 ). However, the use of plant protein sources for carnivorous fish species such as salmonids is still met with challenges as certain plant components may interfere with fish health and welfare( Reference Krogdahl, Penn and Thorsen 4 ). It is well known that increasing levels of plant protein in fish diets will reduce the levels of dietary cholesterol. Traditional marine-based diets will provide at least 1 g cholesterol/kg of feed, whereas plant products contain virtually no cholesterol( Reference Tocher, Bendiksen and Campbell 5 ). Additionally, certain plant components and antinutritional factors (phytosterols and saponins) may impair cholesterol uptake from the intestinal lumen and thereby further decrease plasma cholesterol levels( Reference Krogdahl, Penn and Thorsen 4 , Reference Francis, Makkar and Becker 6 ). Accordingly, inclusion of plant protein in fish feeds typically results in decreased lipid digestibility, hypocholesterolaemia and reduced bile salt levels( Reference Kaushik, Cravedi and Lalles 7 – Reference Yamamoto, Suzuki and Furuita 12 ). A reduction in feed efficiency, fish growth and pathogen resistance is often observed in parallel to alterations in sterol metabolism( Reference Maita, Satoh and Fukuda 13 – Reference Yun, Mai and Zhang 15 ).
Cholesterol is not considered to be an essential nutrient in fish primarily owing to the fact that vertebrates can synthesise cholesterol from acetate. The potential for cholesterol synthesis may vary, however, and the possibility that fish have less capacity than mammals to compensate for a low intake of cholesterol with increased synthesis must be considered. The relatively few reported studies on cholesterol supplementation to aquafeeds suggest that the effects of dietary cholesterol depend on the nature of the basal diet. Dietary cholesterol supplementation (1·0 %) to fishmeal-based diets has been found to not affect growth in Atlantic salmon( Reference Bjerkeng, Storebakken and Wathne 16 ), hybrid striped bass( Reference Sealey, Craig and Gatlin 17 ) and Japanese flounder( Reference Deng, Mai and Ai 18 ). By contrast, cholesterol supplementation has been observed to significantly improve growth when included in soyabean meal-based diets of channel catfish( Reference Twibell and Wilson 19 ), turbot( Reference Yun, Mai and Zhang 15 , Reference Yun, Ai and Mai 20 ) and rainbow trout( Reference Deng, Bi and Kang 21 ) or when included in Salicornia bigelovii seed meal of Nile tilapia( Reference Rios-Duran, Valencia and Ross 22 ). Furthermore, cholesterol supplementation (0·9–1·2 %) to a soyabean meal-based diet has also been found to improve the immune response and resistance to Aeromonas hydrophila in rainbow trout( Reference Deng, Kang and Tao 23 ). This suggests that the negative effects induced by plant components can be attenuated by dietary supplementation with cholesterol. Therefore, cholesterol supplementation may be beneficial when fish are fed plant protein-based diets.
Cholesterol is an integral component of cell membranes and also serves as a precursor to important metabolites such as steroid hormones and bile acids. At present, the regulation of cholesterol metabolism and homeostasis in fish is largely unknown. Cholesterol can be obtained from the diet or synthesised de novo, and the conversion of cholesterol into bile acids represents a major route for the elimination of excess cholesterol from the body. The synthesis of cholesterol from acetyl-CoA involves more than twenty enzymatic reaction steps, but the presumed rate-limiting step is the synthesis of mevalonate by 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). The rate-limiting step in primary bile acid synthesis, and thereby cholesterol catabolism, is mediated by cholesterol 7α-hydroxylase (CYP7A1). Recent studies in fish have reported that high levels of supplemental cholesterol (1–1·5 %) induce CYP7A1 activity and reduce HMGCR activity( Reference Yun, Mai and Zhang 15 , Reference Deng, Bi and Kang 21 ). This indicates that excess levels of cholesterol lead to the suppression of cholesterol biosynthesis and increased conversion into bile acids. On the other hand, several studies( Reference Kortner, Gu and Krogdahl 8 , Reference Maita, Maekawa and Satoh 14 , Reference Geay, Ferraresso and Zambonino-Infante 24 , Reference Vilhelmsson, Martin and Medale 25 ) have reported that high inclusion levels of plant protein stimulate cholesterol biosynthesis, suggesting that the replacement of fishmeal with plant protein sources may result in an inadequate supply of cholesterol in fish. Another crucial factor for controlling cholesterol homeostasis is the uptake of dietary cholesterol from the intestinal lumen( Reference Ikonen 26 ), but essentially nothing is known about these mechanisms in fish.
Plants now supply more than 50 % of protein in Norwegian salmon aquafeeds, but, to our knowledge, there are no reported studies on cholesterol supplementation to plant-based feeds in Atlantic salmon. The present study is a part of a larger experiment investigating dietary supplementation to plant meal-based diets in Atlantic salmon. In the present study, we evaluated the effects of dietary cholesterol supplementation (1·5 %) in Atlantic salmon fed a plant-based diet for 77 d. A comprehensive analytical package was used to shed light on the essentially unexplored pathways of sterol metabolism in fish. The weights of body, intestines and liver were recorded and blood, tissues, faeces, intestinal chyme and bile were sampled for the evaluation of effects on growth, nutrient utilisation and metabolism, and transcriptome and metabolite levels, with particular emphasis on sterol metabolism and organ structure and function. Tissues of interest for the molecular studies were the liver, which is the main organ for cholesterol and bile acid synthesis, and the pyloric caeca, which is the major site of lipid absorption in Atlantic salmon. We hypothesised that dietary supplementation of 1·5 % cholesterol to a plant-based diet would lead to alterations in Atlantic salmon sterol levels that would be reflected in transcriptional and biochemical signatures, as well as growth and nutrient utilisation.
Materials and methods
Experimental animals, diet and sampling
The present study was conducted in compliance with laws regulating experimentation with live animals in Norway as overseen by the Norwegian Animal Research Authority (FDU). The feeding trial was carried out at the Nofima research station at Sunndalsøra, Norway. Atlantic salmon (Salmo salar L.) post-smolts of the Sunndalsøra breed with a mean weight of 362 (sd 95) g were weighed, pit tagged and randomly allocated to four cylindrical fibreglass tanks (200 litres, thirty-five fish/tank) with flow-through seawater (6–7 l/min). In the trial, two replicate tanks per diet were used. Water temperature varied between 7 and 14°C. Oxygen content and salinity of the outlet water were monitored to secure saturation above 85 % and stability, respectively. A 24 h lighting regimen was employed during the experimental period. The fish were weighed individually during allocation to the experimental units to ensure similar biomass in all the tanks. The fish were fed continuously and approximately 20 % in excess of the expected feed intake a plant-based diet with and without 1·5 % cholesterol (Table 1) using automatic disc feeders. Feed intake was not recorded. Diets were formulated to contain 41 % crude protein and 30 % lipid (DM basis). They were supplemented with a standard vitamin and micro-mineral premix and limiting essential amino acids (lysine and methionine) as necessary to provide required amounts as suggested by the NRC guidelines( 27 ). The diets also contained 50 mg/kg yttrium oxide as an inert marker for the calculation of nutrient apparent digestibilities. The formulation and chemical analysis results of the control diet are given in Table 1. Feed was produced by extrusion at the BioMar A/S production facility in Brande, Denmark. Diets were extruded with a feed pellet size of 6 mm. The feeding trial was carried out for 77 d. Tank sampling and fish sampling were conducted randomly. To ensure intestinal exposure to the diets, only fish with digesta throughout the intestinal tracts were sampled. A total of fifteen fish were sampled from each tank and killed by anaesthetisation with tricaine methanesulfonate (MS-222) followed by a sharp blow to the head. Blood was sampled by venepuncture of the caudal vein of ten fish per tank. Blood was collected in vacutainers containing lithium heparin and stored on ice until centrifugation. Plasma was separated and immediately frozen in liquid N2 and stored at − 80°C until analysis. After blood withdrawal, the fish were dissected to remove the viscera. Bile was collected directly from the gall bladder of ten fish per tank using a syringe, transferred to Eppendorf tubes, frozen in liquid N2 and stored at − 80°C until analysis. Intestinal contents (digesta) were collected from the pyloric intestine, mid-intestine and distal intestine. The pyloric and distal intestine contents were divided into proximal (PI1 and DI1, respectively) and distal (PI2 and DI2, respectively) portions. Intestinal contents were frozen in liquid N2 and stored at − 80°C until analysis. Pyloric caeca, mid-intestine and distal intestine, and liver were sampled from five fish per tank for histology and RNA analysis. For histology, tissue samples were fixed in 10 % neutral buffered formalin (4 % formaldehyde) for 24 h and subsequently transferred to 70 % EtOH for storage until processing. For RNA analysis, samples were rinsed in sterile saline and submerged in RNAlater® and stored at 4°C for 24 h and then at − 40°C until analysis. The remaining fish in each tank were stripped for faeces and continued to be fed the feed for an additional week during which they were stripped again. Faecal samples were pooled and frozen until analysis.
Table 1 Formulation and chemical analysis results of the control diet*
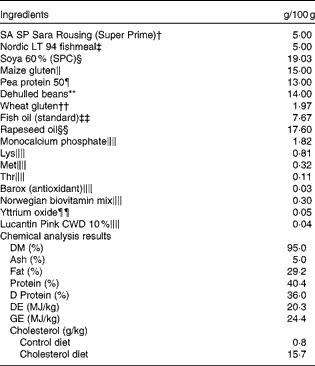
SPC, soya protein concentrate; DE, digestible energy; GE, gross energy.
* The cholesterol diet was identical to the control diet, except for 1·5 % cholesterol. Chemical analysis was conducted only for the control diet, but cholesterol content analysis was conducted for both the diets.
† Supplied by Köster Marine Proteins GmbH.
‡ Supplied by Norsildmel AS.
§ Supplied by Selecta S/A, Avenida Jamel Ceilio, 2496 – 12th region.
∥ Supplied by Cargill Nordic.
¶ Supplied by DLG Food Grain.
** Supplied by HC Handelscenter.
†† Supplied by Roquette.
‡‡ Supplied by FF Skagen.
§§ Supplied by Emmelev.
∥∥ Supplemented to meet the requirements.
¶¶ Inert marker for the evaluation of nutrient digestibility.
RNA extraction
Total RNA was extracted from liver and pyloric caeca samples (approximately 30 mg) using TRIzol® reagent (Invitrogen) and purified with PureLink (Invitrogen) including an on-column DNAse treatment according to the manufacturer's protocol. The integrity of the RNA samples was verified using the 2100 Bioanalyzer in combination with RNA Nano Chip (Agilent Technologies), and RNA purity and concentrations were measured using the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies). RNA samples with integrity number >8 were included in the gene expression analysis. Total RNA samples were stored at − 80°C until use.
Microarray analyses
Microarray analyses were carried out on liver samples. A two-colour design was used, where individual fish samples (five in each study group, two to three fish from each tank duplicate) were labelled with fluorescent Cy3 and hybridised against a common reference sample labelled with fluorescent Cy5. The common reference sample consisted of a pool of ten individual fish fed a fishmeal-based diet. Nofima's Atlantic salmon 15k oligonucleotide microarray SIQ-6 (GEO Omnibus GPL16555) was obtained from Agilent Technologies and, unless indicated otherwise, the reagents and equipment were also from the same source. RNA amplification and labelling were performed using the Two-Colour Low Input Quick Amp Labelling Kit and the Gene Expression Hybridisation Kit was used for the fragmentation of labelled RNA. The amount of total RNA used in each reaction was 200 ng. After overnight hybridisation in an oven (17 h at 65°C and rotation speed of 10 rpm), arrays were washed with Gene Expression Wash Buffers 1 and 2 and scanned with GenePix 4100A (Molecular Devices). GenePix Pro 6.0 was used for spot-to-grid alignment, spot quality assessment, feature extraction and quantification. Subsequent data analyses were carried out using the bioinformatic system STARS( Reference Krasnov, Timmerhaus and Afanasyev 28 ). After filtration of low-quality spots flagged by GenePix, Lowess normalisation of log2-expression ratios was performed. Genes that passed quality control in at least four samples per group were included in subsequent analyses. Differentially expressed genes were selected based on the following criteria: fold difference >1·6 and P< 0·05 (t test). The enrichment of Gene Ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) terms in the list of differentially expressed genes was assessed with Yates' corrected χ2 using all the probes that passed quality control as a reference; enriched terms corresponding to at least five differentially expressed genes were selected. Complete data files were deposited at the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus with accession no. GSE51887.
Quantitative real-time PCR
Hepatic gene expression was quantified by quantitative real-time PCR (qRT-PCR) to validate microarray analysis results and to examine particular genes of interest in detail. In addition, qRT-PCR was used for the quantification of genes related to lipid and sterol metabolism in pyloric caeca. Assays were carried out according to the MIQE (Minimum Information for Publication of Quantitative Real-Time PCR Experiments) standards( Reference Bustin, Benes and Garson 29 ) on ten fish from each diet group (five fish from each tank duplicate). First-strand complementary DNA was synthesised using 0·8 μg of total RNA from all samples using Superscript III (Invitrogen) in 20 μl reaction mixtures and primed with oligo(dT)20 primers according to the manufacturer's protocol. Negative control tests were carried out in parallel by omitting RNA or enzyme. The obtained complementary DNA was diluted 1:10 before use and stored at − 20°C. The qRT-PCR primers were designed based on the literature or using Primer3 (http://frodo.wi.mit.edu/primer3/). Primer details are given in online supplementary Table S1. All primer pairs gave a single band pattern for the expected amplicon of interest in all the reactions. PCR efficiency for each gene assay was determined using 10-fold serial dilutions of randomly pooled complementary DNA. The expression of individual gene targets was analysed using the LightCycler 480 (Roche Diagnostics). Each 10 μl DNA amplification reaction mixture contained 2 μl PCR-grade water, 2 μl of 1:10 diluted complementary DNA template, 5 μl of LightCycler 480 SYBR Green I Master (Roche Diagnostics) and 0·5 μl (final concentration 500 nm) of each forward and reverse primer. Each sample was assayed in duplicate, including a no-template control. The three-step qRT-PCR programme included an enzyme activation step at 95°C (5 min) and forty cycles of 95°C (10 s), 60°C (10 s) and 72°C (15 s). Quantification cycle (C q) values were calculated using the second derivative method. To confirm amplification specificity, the PCR products from each primer pair were subjected to melting curve analysis and visual inspection after each run by agarose gel electrophoresis. For target gene normalisation, actb (β-actin), ef1a (elongation factor 1α), gapdh (glyceraldehyde-3-phosphate dehydrogenase) and rnapolII (RNA polymerase II) were evaluated for use as reference genes by ranking relative gene expression according to their overall CV and their interspecific variance, as described previously( Reference Kortner, Valen and Kortner 30 ). For pyloric caeca samples, gapdh was used as a normalisation factor, whereas the geometric average of gapdh, actb and rnapolII was used for liver samples. The relative expression of target genes was calculated using the ΔΔ C t method( Reference Livak and Schmittgen 31 ).
Chemical analysis
Diet and faecal samples were analysed for DM (after heating at 105°C for 16–18 h), ash (by combusting at 550°C to constant weight), nitrogen (crude protein) (using the semi-micro Kjeldahl method, Kjeltec Auto System; Tecator), fat (by diethyl ether extraction in a Fosstec analyser (Tecator) after HCl hydrolysis), starch (measured as glucose after hydrolysis by α-amylase (Novo Nordisk A/S) and amyloglucosidase (Boehringer Mannheim GmbH), followed by determination of glucose levels using the ‘Glut-DH method’ (Merck)), gross energy using the Parr 1271 Bomb calorimeter (Parr) and yttrium by inductively coupled plasma MS as described by Refstie et al. ( Reference Refstie, Helland and Storebakken 32 ). The levels of amino acids in the diet samples were determined using a Biochrom 30 amino acid analyser following the EC Commission Directive 98/64/EC (1999) after hydrolysis in 6 m-HCl for 23 h at 110°C. The levels of tryptophan and tyrosine were determined after basic hydrolysis. For analyses of fatty acid (FA) composition, lipid extracts were transmethylated over night with 2,2-dimethoxypropane, methanolic HCl and benzene at room temperature, as described by Mason & Waller( Reference Mason and Waller 33 ) and Hoshi et al. ( Reference Hoshi, Williams and Kishimoto 34 ). The methyl esters of the FA thus formed were separated in a gas chromatograph (Hewlett Packard 6890) with a split injector, a SGE BPX70 capillary column (length 60 m and internal diameter 0·25 mm and thickness of the film 0·25 μm) and a flame ionisation detector, and the results were analysed using the HP ChemStation software. Helium was used as the carrier gas. The injector and detector temperatures were 280°C. The oven temperature was raised from 50 to 180°C at a rate of 10°C/min and then raised to 240°C at a rate of 0·7°C/min. The relative quantity of each FA present was determined by measuring the area under the chromatograph peak corresponding to that FA.
Plasma variables and bile salt levels in bile and intestinal contents
Plasma samples were analysed for NEFA, cholesterol, total TAG and glucose following standard procedures at the Central Laboratory of the Norwegian School of Veterinary Science (NVH), Oslo. The levels of total intestinal bile salts were measured in pooled freeze-dried gastrointestinal contents from the PI1, PI2, MI, DI1, and DI2 portions. The levels of bile salts were determined using the enzyme cycling amplification/thionicotinamide-aderine dinucleotide (Thio-NAD) method (Inverness Medical) in the ADVIA®1650 Chemistry System (Siemens Healthcare Diagnostics, Inc.) at the Central Laboratory of NVH. In bile taken directly from the gall bladder, the levels of glycine- and taurine-conjugated bile acids were determined using HPLC–MS/MS by a modification of the method described by Tagliacozzi et al. ( Reference Tagliacozzi, Mozzi and Casetta 35 ) using 2H-labelled glycine derivatives of bile acids as internal standards. In some cases, the levels of bile acids were also determined by isotope dilution and combined GC–MS after deconjugation as described by Björkhem & Falk( Reference Björkhem and Falk 36 ). The plasma levels of oxysterol were determined by isotope dilution and combined GC–MS after hydrolysis as described by Dzeletovic et al. ( Reference Dzeletovic, Breuer and Lund 37 ). The levels of sitosterol and campesterol were determined by isotope dilution and combined GC–MS after hydrolysis as described by Acimovic et al. ( Reference Acimovic, Lovgren-Sandblom and Monostory 38 ). The levels of lathosterol were determined by isotope dilution MS as described by Lund et al. ( Reference Lund, Sisfontes and Reihner 39 ). The levels of 7α-hydroxy-4-cholesten-3-one (C4) were determined by isotope dilution and combined HPLC–MS as described by Lövgren-Sandblom et al. ( Reference Lövgren-Sandblom, Heverin and Larsson 40 ). Plasma lipoprotein profiles were determined employing size exclusion chromatography and measurements of cholesterol and TAG on-line using microlitre sample volumes as described by Parini et al. ( Reference Parini, Johansson and Broijersén 41 ).
Histology
Samples used for histology were processed using standard histological techniques and stained with haematoxylin and eosin at the NVH. Pyloric caeca and liver tissue samples were evaluated by light microscopy in a randomised order.
Calculations
Crude protein content was calculated as N × 6·25. Thermal growth coefficient (TGC) was calculated as follows:
where IBW and FBW are the initial and final body weights (tank means) and ΣD° is the thermal sum (feeding days × average temperature in °C). Organosomatic indices were calculated as the percentages of the weight of the organ in relation to body weight. Apparent digestibility (AD) was estimated by the indirect method using Y2O3 as an inert marker and calculated as follows:
where M feed and M faeces are the percentage concentration of the inert marker (Y2O3) in the feed and faeces, respectively, and N feed and N faeces are the percentage concentration of a nutrient in the feed and faeces, respectively. Nutrient retention (retentions of crude protein, individual amino acids and energy) was calculated as follows:
where C diet is the nutrient content in the diets and C 0 and C 1 are the initial and final nutrient contents in the fish.
Statistical analyses
Statistical analyses were carried out using GraphPad Prism version 6.03 (GraphPad Software, Inc.). The feeding trial had a completely randomised design, and cholesterol inclusion was evaluated as the class variable. Data were analysed using a two-sided Student's t test with a significance level of P< 0·05, unless otherwise indicated. qRT-PCR data are presented as P< 0·05 or NS (P>0·05); for all other data, the actual P values are reported.
Results
Fish growth, organ indices, nutrient digestibilities and histology
Data on fish growth and nutrient digestibilities are given in Table 2. Cholesterol supplementation did not affect the growth rates or macronutrient digestibility. Nor did it affect the digestibility of individual FA or amino acids (data not shown). A trend towards reduced taurine ‘digestibility’ was observed, indicating increased endogenous output of taurine-containing compounds to the intestine. Organ indices and histomorphology of the pyloric caeca and liver were not affected (data not shown).
Table 2 Mean final body weights, thermal growth coefficients (TGC), specific growth rates (SGR) and apparent digestibilities (Mean values with their standard errors)
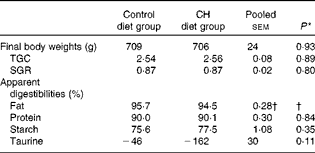
CH, cholesterol.
* P values obtained in two-sided Student's t test are given.
† Statistical test could not be conducted due to one missing value. The mean value reported for fat digestibility is the pooled means with their standard errors for all the twenty-three tanks included in the feeding trial.
Blood plasma biochemistry
Data on blood plasma biochemistry are given in Table 3. Cholesterol supplementation to the plant-based diet resulted in the expected, marked elevation of plasma cholesterol levels. On the other hand, cholesterol supplementation reduced blood plasma TAG levels. The pattern and distribution of lipids in the three lipoprotein fractions (HDL, LDL and VLDL) were similar to those in rodents in the control group. Thus, most of the cholesterol and TAG were distributed in the HDL fraction. There was a pronounced change in the pattern and distribution of lipids in the plasma of fish fed the cholesterol-containing diet (Table 3 and Fig. 3). In these fish, most of the increase in cholesterol levels seemed to occur in the LDL fraction, showing a 10-fold increase, whereas the levels in the HDL fraction seemed to be unaffected. The levels of TAG in the HDL fraction were reduced, and most of the TAG were distributed in the LDL and VLDL fractions. Cholesterol supplementation did not affect plasma bile salt levels. No effects of cholesterol supplementation were observed on plasma glucose or free FA levels (data not shown). The cholesterol-containing diet decreased the circulating lathosterol levels, indicative of decreased cholesterol synthesis in the liver. C4 is an intermediate in bile acid synthesis, and the plasma level of this oxysterol has been shown to be a marker of bile acid synthesis in humans( Reference Axelson, Björkhem and Reihnér 42 ). Thus, it seemed likely that the high levels of C4 in the cholesterol-supplemented group reflected the markedly increased synthesis of bile acids as a consequence of the dietary load of cholesterol. The levels of the precursor of C4, 7α-hydroxycholesterol, were also substantially increased in the plasma of fish fed the cholesterol-containing diet. Cholesterol supplementation resulted in increased plasma levels of 7β-hydroxycholesterol and 7-keto-cholesterol as well as 24-, 25- and 27-hydroxycholesterol. Cholesterol supplementation also seemed to abolish the intestinal uptake of plant sterols, as evidenced by the markedly reduced plasma levels of sitosterol and campesterol.
Table 3 Mean blood plasma variables (Mean values with their standard errors (n 10 fish per diet group))
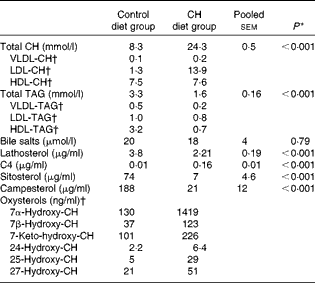
CH, cholesterol; C4, 7α-hydroxy-4-cholesten-3-one.
* P values obtained in two-sided Student's t test are given.
† Lipoprotein and oxysterol profiles were measured in a pooled sample of ten fish per diet group. CV for the different assays, i.e. the analytical variance, as estimated by analysing a control sample over ten consecutive days, were as follows: VLDL-CH: 8·1 %; LDL-CH: 3·4 %; HDL-CH: 5·0 %; VLDL-TAG: 13·1 %; LDL-TAG: 10·5 %; HDL-TAG: 9·7 %. CV for all oxysterol assays were < 8 %, except for 25-hydroxy-CH assay (11 %)( Reference Dzeletovic, Breuer and Lund 37 ).
Gall bladder and chyme bile acid levels
Data on gall bladder and chyme bile acid levels are given in Table 4. No significant differences in total bile acid levels or total conjugated bile acid levels were found between the diet groups. The majority of bile acids in the gallbladder bile were conjugated; the only unconjugated bile acid found was cholic acid, which was detected at low concentrations. However, cholic acid levels were significantly increased after cholesterol supplementation. The taurine-conjugated bile acids were the predominant form of bile acids found in the bile, with taurocholic acid being the predominant individual bile acid. While no diet effect was observed on taurocholic acid levels, taurochenodeoxycholic acid levels were higher in the bile of fish fed the cholesterol-containing diet. The glycine-conjugated bile acids were detected at very low concentrations, and there were no differences in the levels between the diet groups. In the intestinal chyme, bile salt levels decreased gradually from the proximal to the distal parts. No statistically significant differences in the levels in any of the intestinal regions were observed between the diet groups.
Table 4 Mean bile acid levels in the gall bladder and gut content (Mean values with their standard errors, n 10 fish per group)
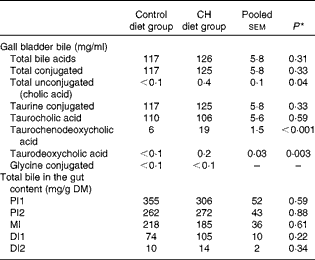
CH, cholesterol; PI1, pyloric intestine portion 1; PI2, pyloric intestine portion 2; MI, mid-intestine; DI1, distal intestine portion 1; DI2, distal intestine portion 2.
* P values obtained in two-sided Student's t test are given.
Intestinal gene expression
The pyloric caeca expression profiles of key genes involved in lipid and sterol transport and biosynthesis and transcriptional control are shown in Fig. 1. Except for a significant down-regulation of FA transporter cd36 (cluster of differentiation 36) expression, cholesterol supplementation exerted limited effects on the expression of genes involved in fat absorption and turnover and lipoprotein synthesis (fatp (FA transport protein), fabp2a1 (FA-binding protein 2a1), mgat2a (monoacylglycerol acyltransferase 2-A), mtp (microsomal triglyceride transfer protein) and apo). By contrast, cholesterol supplementation markedly increased the mRNA levels of the basolateral (abca1) and apical (abcg5) cholesterol efflux transporters and decreased the mRNA levels of Niemann–Pick C1-like 1 (npc1l1), presumably responsible for cholesterol uptake from the intestinal lumen. There was an increase in the expression of acyl-CoA cholesterol acyltransferase (acat), witch catalyses the intracellular esterification of cholesterol. There was an increase in the expression of taurine transporter slc6a6 in fish fed the cholesterol-containing diet, whereas no effects were observed on the expression of hmgcr. Cholesterol supplementation resulted in a differential expression of several nuclear receptors with important regulatory roles in fat and sterol homeostasis. There was an increase in the expression of oxysterol-activated transcription factor liver X receptor (lxr) as well as sterol element-binding protein 1 (srebp1) and pparα. By contrast, cholesterol supplementation led to a decrease in srebp2 mRNA levels.

Fig. 1 Gene expression profiling of (a) liver and (b) pyloric caeca samples by quantitative real-time PCR (qRT-PCR). Values are mean ΔΔ C t, with their standard errors represented by bars (n 10 fish per group). Mean values obtained for liver samples of the cholesterol-supplemented group were significantly different from those of the control group, except for those marked with NS (P< 0·05). * Mean values obtained for pyloric caeca samples of the cholesterol-supplemented group were significantly different from those of the control group, (P< 0·05). † Gene expression was also measured by microarray analyses. See online Supplementary Table S1 for a list of names and abbreviations.
Hepatic gene expression
The microarray analysis and qRT-PCR data were closely correlated (Pearson's correlation coefficient: 0·90, P< 0·0001). In the microarray analyses, 322 entities were found to be to be differentially expressed between the two diet groups (fold change >1·6, P< 0·05). A search for enriched Gene Ontology terms and KEGG pathways in the list of differentially expressed genes was carried out for rapid screening of the thematic associations of the transcriptional response (Table 5). As expected, the results indicated that cholesterol supplementation led to alterations in sterol biosynthesis pathways (steroid, steroid hormone and terpenoid synthesis). This observation was reflected in the individual gene expression signatures (see Table 6 for microarray analysis results and Fig. 1 for qRT-PCR data). The effect of cholesterol supplementation was evident, as we observed a strong and concerted down-regulation of the expression of nearly all enzymes involved in cholesterol biosynthesis (Fig. 2) as well as the master regulator srebp2. The microarray analysis results indicated the down-regulation of the expression of genes that take part in biotransformation and enhance the solubility of steroids (sts (steroid sulfatase, microsomal isozyme), gstt1b (glutathione S-transferase θ 1) and cyp1a1 (cytochrome P450 1A1)). Furthermore, increased conversion of cholesterol into bile acids was indicated by an astonishing 400-fold increase in cyp7a1 expression after cholesterol supplementation. Bile production was also indicated by the up-regulation of the expression of cyp27a1 (cytochrome P450 27A1), two isoforms of the taurine transporter slc6a6, abcc2 (multidrug resistance-associated protein 2), a mediator of hepatobiliary excretion of diverse organic anions, and cysteine sulfinate decarboxylase (csd), which is involved in taurine biosynthesis. The induction of alcohol dehydrogenase 3 (adh3) expression is also worth mentioning, as this enzyme catalyses the oxidation of intermediary alcohols of the shunt pathway of mevalonate metabolism, which provides a biochemical link between cholesterol and leucine metabolism( Reference Keung 43 ). Furthermore, the expression of lxr was induced together with that of its obligate partner retinoid X receptor (rxr), whereas that of abcg5 was stable. The expression of several lipoproteins was induced in parallel to that of lipoprotein lipase (lpl) and cholesteryl ester transfer protein (cetp), which facilitates the transport of cholesterol esters and TAG between lipoproteins. The expression of a suite of genes encoding enzymes involved in lipid metabolism was down-regulated in concert with that of peroxisomal biogenesis factor 7 (wdr77). However, there was an increase in the expression of three regulators of adipocyte differentiation (cebpg (CCAAT/enhancer-binding protein-γ), cebpb (CCAAT/enhancer-binding protein-β2) and adfp (adipophilin)).
Table 5 Functional Gene Ontology (GO) categories and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways enriched with genes that were differentially expressed (DEG) in response to cholesterol supplementation
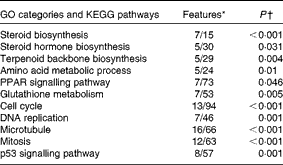
* Numbers of genes among DEG and on the microarray platform.
† Yates' corrected χ2.
Table 6 Differentially expressed genes involved in steroid, bile and lipid metabolism (hepatic microarray analysis)*

* Values are mean fold change observed in the cholesterol diet-fed group in comparison with those in the control diet-fed group.
† P values obtained in the t test are given.
‡ Genes were confirmed by quantitative real-time PCR (see Fig. 2).

Fig. 2 Regulation of hepatic cholesterol biosynthesis by dietary cholesterol supplementation. Major metabolic intermediates are shown in red font and genes are shown in black font. Microarray (MA) and quantitative real-time PCR (qPCR) values are mean fold change observed in the cholesterol-supplemented group in comparison with those in the control group. * Mean values were not significantly different (P>0·05). SREBP-2, sterol regulatory element-binding protein 2; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; GPP, geranyl diphosphate; IPP, isopentenyl diphosphate; FPP, farnesyl pyrophosphate; C4, 7α-hydroxy-4-cholesten-3-one; CYP51, cytochrome P450 family 51; DHCR7, 7-dehydrocholesterol reductase; CYP7A1, cytochrome P450 7A1.
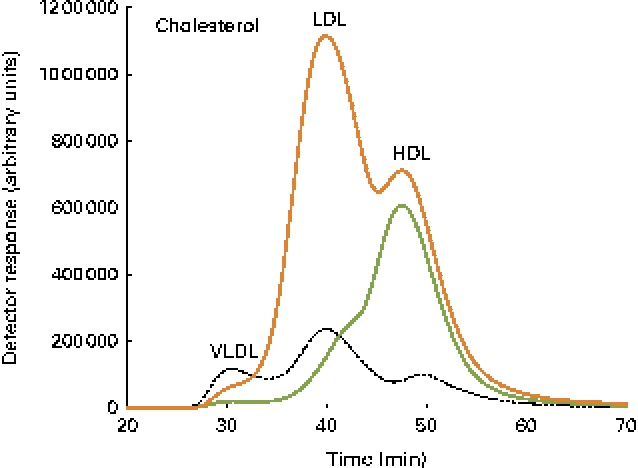
Fig. 3 Cholesterol lipoprotein profiles. A pooled sample of ten fish per diet group, control ![]() and cholesterol
and cholesterol ![]() , as well as a human plasma sample
, as well as a human plasma sample ![]() , was used, and the elution peaks of the main lipoproteins are shown.
, was used, and the elution peaks of the main lipoproteins are shown.
Cholesterol supplementation seemed to affect cell proliferation and cell-cycle progression as reflected by the enrichment of terms related to DNA replication, mitosis and cell cycle (Table 5). This was manifested in individual gene signatures by a concerted down-regulation of the expression of genes related to DNA replication, nucleotide biosynthesis, and chromosome maintenance, spindle formation, and chromosome segregation and mitosis (Table 7). This was in line with the increased expression of the negative regulators of cell proliferation tob1 (Tob1) and cdkn1c (cyclin-dependent kinase inhibitor 1C). The effect of cholesterol supplementations on the hepatic expression of immune genes was relatively small (Table 8). The up-regulation of the expression of C1q components of the classical complement pathways together with that of fcer1 g (high-affinity Ig receptor subunit-γ), which binds to opsonised antibodies, is worth mentioning. The expression of four mediators of inflammation including that of three isoforms of lect2 l (leukocyte cell-derived chemotaxin 2-1) was down-regulated.
Table 7 Differentially expressed genes involved in cell proliferation (hepatic microarray analysis)*

* Values are mean fold change observed in the cholesterol diet-fed group in comparison with those in the control diet-fed group.
† P values obtained in the t test are given.
Table 8 Differentially expressed genes involved in immunity (hepatic microarray analysis)*

* Values are mean fold change observed in the cholesterol diet-fed group in comparison with those in the control diet-fed group.
† P values obtained in the t test are given.
Discussion
In the present study, dietary cholesterol supplementation (1·5 %) to a plant-based diet was found to not affect Atlantic salmon growth, macronutrient digestibility, organ indices, or hepatic and intestinal histomorphology. Cholesterol supplementation has been reported to significantly improve fish growth when included in diets containing high levels of soyabean meal( Reference Yun, Mai and Zhang 15 , Reference Twibell and Wilson 19 – Reference Deng, Bi and Kang 21 ). The lack of an effect in the present study is most probably due to the inclusion of more refined plant protein sources such as soya protein concentrate and wheat gluten, which probably reduced the dietary load of plant antinutrients. On the other hand, the present study clearly demonstrated how Atlantic salmon adjusted their metabolic functions in response to the dietary load of cholesterol. It has also expanded our understanding of sterol metabolism and turnover, adding to the existing, rather sparse, knowledge of these processes in fish. The results are outlined in the following sections and compared with the current understanding of sterol metabolism in humans and rodents when applicable.
Intestinal cholesterol absorption
Whole-body cholesterol homeostasis is maintained by the regulation of cholesterol absorption from the intestinal lumen, endogenous cholesterol biosynthesis, conversion of cholesterol into bile acids, and faecal excretion of bile. Although the liver has traditionally been considered to be the major site of control in the maintenance of cholesterol homeostasis, the importance of the intestine in many aspects of cholesterol physiology is increasingly recognised( Reference van der Wulp, Verkade and Groen 44 , Reference Wang 45 ). Cholesterol present in the intestinal lumen derives from several sources, including diet, bile, intestinal secretion and shed epithelial cells. The molecular mechanisms responsible for sterol uptake have not been completely defined, but the intestinal uptake of cholesterol and plant sterols is believed, at least partially, to be mediated by the NPC1L1 transporter( Reference Davis, Zhu and Hoos 46 ). Cholesterol that is absorbed or synthesised locally in the enterocytes is thought to be either expelled into the intestinal lumen through the actions of the ATP-binding cassette ABCG5/ABCG8 heterodimeric transporter for eventual excretion via the faeces or esterified by the action of ACAT and packaged into chylomicrons for transport into the body( Reference Brunham, Kruit and Iqbal 47 ). In addition, the current literature indicates that cholesterol may be exported out to the basolateral compartment in HDL particles via the ABCA1 efflux pump( Reference van der Wulp, Verkade and Groen 44 , Reference Hui, Labonte and Howles 48 ). The efflux of plant sterols from enterocytes back to the intestinal lumen is facilitated by ABCG5/G8( Reference Calpe-Berdiel, Escola-Gil and Blanco-Vaca 49 ). In the present study, gene expression profiling indicated that the dietary load of cholesterol promoted the regulation of intracellular sterol levels in the intestine by reducing sterol uptake and inducing sterol efflux. First, the expression of npc1l1, presumably responsible for cholesterol uptake, was suppressed, whereas that of the apical (abcg5) and the basolateral (abca1) efflux transporters was induced. Second, increased capacity for cholesterol esterification was indicated by the increased acat mRNA levels. In addition to cholesterol assembly into lipoproteins, cholesterol esterification may function as an intracellular buffering mechanism for the storage of FA sterol esters in lipid droplets. Cholesterol supplementation also seemed to abolish the intestinal uptake of plant sterols. The very low plasma levels of sitosterol and campesterol found in the fish fed the cholesterol-containing diet are likely to reflect a competition between cholesterol and the plant sterols in the intestine, as well as a result of alterations in sterol transport mechanisms( Reference Liland, Espe and Rosenlund 50 ). In parallel to alterations in the expression of sterol transporters, we observed the differential expression of several nuclear receptors with important regulatory roles in lipid and sterol metabolism. Specifically, there was a decrease in the expression of srebp2 after cholesterol supplementation, whereas the expression of lxr, srebp1 and pparα was induced. The induction of lxr expression was not unexpected, as LXR is activated by oxysterols and is known to induce abcg5/abcg8 and abca1 expression in mammals( Reference Kalaany and Mangelsdorf 51 ). Additionally, SREBP2 is inactivated by high intracellular cholesterol levels( Reference Horton, Goldstein and Brown 52 ), and srebp2 has recently been reported as a master transcriptional regulator of cholesterol metabolism in Atlantic salmon( Reference Leaver, Villeneuve and Obach 53 ). The suppression of srebp2 expression by the dietary load of cholesterol probably reflects the suppression of endogenous cholesterol biosynthesis, as outlined below. The induction of pparα expression indicates a similar effect on cholesterol synthesis, as it is known that the activation of PPARα decreases cholesterol synthesis by reduction of nuclear SREBP-2 levels( Reference Konig, Koch and Spielmann 54 ).
Cholesterol transport in plasma
Cholesterol and cholesteryl esters are transported in the blood in the form of lipoproteins. In mammals( Reference Ikonen 26 ), dietary cholesterol is transported from the intestine to the liver in the form of chylomicrons. In turn, hepatocytes secrete lipids in VLDL particles that are processed in the circulation into LDL, which deliver cholesterol to extrahepatic tissues. Finally, extrahepatic tissues can release excess cholesterol in HDL particles, which returns to the liver. In the present study, a pronounced change in the pattern and distribution of lipids in the plasma of fish fed the cholesterol-containing diet was observed, and the profile was found to be similar to the ‘atherogenic’ profile found in hypercholesterolaemic patients. CETP is a plasma protein that facilitates the transport of cholesteryl esters and TAG between lipoproteins. High-cholesterol diets induce both mRNA levels and activity of CETP in a wide range of mammalian species and probably facilitate the recycling of excess cholesterol deposited in extrahepatic tissues( Reference Tall 55 ). The present study demonstrated a similar mechanism in fish, as evidenced by the induction of cetp expression in fish fed the cholesterol-containing diet. Additionally, the expression of lpl was induced, indicative of the selective uptake of cholesterol from the dominating lipoprotein fraction LDL( Reference Merkel, Heeren and Dudeck 56 ).
Cholesterol and bile acid biosynthesis
An obvious effect of cholesterol supplementation was the strong and concerted hepatic down-regulation of the expression of genes encoding nearly all enzymes involved in de novo cholesterol biosynthesis( Reference Horton, Goldstein and Brown 52 , Reference Goldstein, DeBose-Boyd and Brown 57 ), coinciding with the decrease in the expression of the master regulator srebp2 ( Reference Sakakura, Shimano and Sone 58 , Reference Brown and Goldstein 59 ). Furthermore, the expression of both lxr, recently reported to be regulated by oxysterols in rainbow trout( Reference Cruz-Garcia, Sanchez-Gurmaches and Gutierrez 60 ), and its obligate partner rxr was induced by cholesterol supplementation. Gene expression data were further supported by the reduced plasma lathosterol levels in the cholesterol-supplemented group, as the circulating levels of this intermediate in cholesterol synthesis reflect whole-body cholesterol synthesis( Reference Kempen, Glatz and Leuven 61 ). As lathosterol is transported in the same way as cholesterol and dependent on the same transporters, the pool of cholesterol will have some effect on the absolute levels. Thus, it is appropriate to assess the lathosterol:cholesterol ratio. Therefore, the suppression will be even more marked after correction for the cholesterol levels. Interestingly, hmgcr expression was not influenced by cholesterol supplementation. This is in contrast to the current understanding of HMGCR as the rate-controlling enzyme in cholesterol biosynthesis and therefore an important regulatory enzyme( Reference Brown and Goldstein 59 ). However, it should be emphasised that this enzyme is also regulated by post-translation mechanisms and that such mechanisms may be more important than the transcriptional mechanisms( Reference Goldstein, DeBose-Boyd and Brown 57 ). Based on its presumed key regulatory function in cholesterol synthesis, HMGCR has been a subject of targeted investigation in fish feeding trials and seems to be affected by cholesterol supplementation( Reference Deng, Bi and Kang 21 ) or by hypocholesterolaemia induced by plant-based feed( Reference Kortner, Gu and Krogdahl 8 , Reference Maita, Maekawa and Satoh 14 , Reference Gu, Kortner and Penn 62 ). However, several microarray-based approaches have reported the differential expression of a wide range of cholesterol biosynthetic genes in response to plant-based feeding( Reference Geay, Ferraresso and Zambonino-Infante 24 , Reference Leaver, Villeneuve and Obach 53 , Reference Panserat, Hortopan and Plagnes-Juan 63 , Reference Morais, Taggart and Guy 64 ). Thus, it seems likely that cholesterol synthesis in fish is regulated by the transcriptional control of most of the genes involved in the cholesterol biosynthetic pathway, similar to observations made in mammals( Reference Horton, Goldstein and Brown 52 , Reference Sakakura, Shimano and Sone 58 ). The suppression of de novo cholesterol synthesis was also indicated by the induction of adh3 expression. This enzyme catalyses the oxidation of intermediary alcohols of the shunt pathway of mevalonate metabolism, which diverts about 5 % of the production of mevalonate in the rat liver( Reference Keung 43 , Reference Weinstock, Kopito and Endemann 65 ).
Biliary secretion of sterols, either as cholesterol or after conversion into bile salts, is the only significant mechanism for cholesterol removal from the body, and bile acids account for approximately 50 % of the cholesterol lost in humans( Reference Repa and Mangelsdorf 66 ). High dietary cholesterol levels are known to lead to increased synthesis of bile acids in rodents but not in humans, in whom reduction in the intestinal absorption of cholesterol seems to be the main regulatory mechanism( Reference Kalaany and Mangelsdorf 51 , Reference Spady and Cuthbert 67 ). In mice, the cholesterol-induced increase in bile acid synthesis has been shown to involve an LXR-mediated induction of the rate-limiting enzyme in bile acid synthesis, CYP7A1( Reference Peet, Turley and Ma 68 ). In accordance with this, in the present study, increased hepatic mRNA levels of lxr and markedly increased levels of cyp7a1 were observed, suggesting a similar mechanism. Increased CYP7A1 enzymatic activity after dietary cholesterol treatment has also been reported in previous fish studies( Reference Yun, Mai and Zhang 15 , Reference Deng, Bi and Kang 21 ). C4 is an intermediate in bile acid synthesis, and the plasma level of this oxysterol has been shown to be a marker of bile acid synthesis in humans( Reference Axelson, Björkhem and Reihnér 42 ). It seems likely that this is also the case in fish, but to our knowledge this has not been studied previously. Plasma C4 levels in the control fish were low, lower than the corresponding levels in the mouse and man. However, C4 levels in the cholesterol-supplemented group were considerably higher, reflecting the markedly increased synthesis of bile acids as a consequence of the dietary load of cholesterol. In support of this, it was also observed that the levels of the precursor of C4, 7α-hydroxycholesterol, were increased in the plasma of fish fed the cholesterol-containing diet. There was a very high correlation between C4 and 7α-hydroxycholesterol levels (r 0·96). It should be emphasised that the circulating levels of C4 and oxysterols are to some extent affected by the size of the pool of cholesterol in plasma. In view of this, it would be appropriate to compare the C4:oxysterol:cholesterol ratio rather than the absolute levels. Even after such a ‘correction’, the plasma levels of C4 and 7α-hydroxycholesterol were still much higher in the cholesterol-supplemented group, reflecting the high bile acid synthesis. The major pathway for bile acid synthesis in mammals, and most probably also in fish, involves CYP7A1 as a rate-limiting and highly regulated enzyme. In addition, there is a minor pathway (the alternative or acid pathway) involving 27-hydroxylation as the first and rate-liming step( Reference Russell 69 ). This enzyme is not regulated to the same extent as CYP7A1, at least not in mammals( Reference Bjorkhem, Araya and Rudling 70 ). The levels of 27-hydroxycholesterol in the circulation may reflect the activity of this pathway. Dietary cholesterol supplementation increased the plasma levels of 27-hydroxycholesterol as well as hepatic mRNA levels of cyp27a1, suggesting that this pathway for bile acid formation was also stimulated. However, most probably, this increase is secondary to the increased carrier function with high levels of cholesterol, and the 27-hydroxycholesterol:cholesterol ratio was not increased. Increased bile salt production was also indicated by the large negative AD of taurine as well as the induced expression of the taurine transporter slc6a6 in both the liver and intestine. Taken together, these findings strongly suggest that the dietary load of cholesterol resulted in decreased cholesterol synthesis as well as hepatic conversion of excess cholesterol into bile acids. The increased hepatic synthesis of bile acids in fish fed the cholesterol-containing diet was, however, not reflected in total bile acid levels or total conjugated bile acid levels in the gall bladder. The taurine-conjugated bile acids were the predominant form of bile acids found in the bile, with taurocholic acid being the predominant individual bile acid. This is in accordance with previous reports on salmonid bile composition( Reference Bogevik, Tocher and Langmyhr 71 , Reference Denton and Yousef 72 ). While no diet effect on taurocholic acid levels was observed, taurochenodeoxycholic acid levels were higher in the bile of fish fed the cholesterol-containing diet, and unconjugated cholic acid levels were also significantly increased. Further studies using isotope-labelled cholesterol should be conducted to quantify the rate of cholesterol conversion into bile acids.
Effects of dietary cholesterol supplementation on fat metabolism and other cellular functions
The marked reduction in plasma TAG levels in fish fed the cholesterol-containing diet is in accordance with observations made in hypercholesterolaemic mice( Reference Obama, Nagaoka and Akagi 73 ) and indicates that cholesterol and/or its metabolites are involved in the regulation of TAG metabolism in salmon. No effects were observed on the intestinal expression levels of genes related to lipid uptake and transport, except for a decrease in cd36 expression. However, CD36 has also been suggested to be involved in cholesterol uptake from the intestinal lumen in mammals( Reference Nauli, Nassir and Zheng 74 ). Similarly, in the liver, the main responses related to lipid metabolism were, most probably, directly caused by the dietary load of cholesterol (apo-L3 (apo L3), apo-AIV (apo AIV) and lpl). The TAG biosynthetic pathway was not affected, with the exception of a 2-fold induction of 1-acyl-sn-glycerol-3-phosphate acyltransferase-γ (agpat3) expression, which could be a response to the decreased plasma TAG levels. The hepatic gene expression data suggested a decrease in cell proliferation, but neither fish growth nor organ indices were affected. It is possible that the cholesterol-enriched diet induced hypertrophic growth of the liver. Cholesterol supplementation also seemed to affect the non-specific immune system by the up-regulation of the expression of several complement genes. Corresponding observations have been made in rainbow trout, showing that dietary cholesterol supplementation increased the activity of the alternative complement pathway and several other non-specific immune parameters( Reference Deng, Kang and Tao 23 ).
Conclusions
Cholesterol supplementation to a plant-based diet did not affect the growth, organ weights, or intestinal and hepatic histomorphology of Atlantic salmon. On the other hand, dietary cholesterol supplementation seemed to promote the induction of sterol efflux by enterocytes, whereas sterol uptake from the intestine was suppressed. In the liver, the synthesis of cholesterol decreased correspondingly and conversion into bile acids increased. The marked effect of cholesterol supplementation on bile acid synthesis suggests that dietary cholesterol can be used to increase bile acid synthesis in fish. However, the dietary load of cholesterol used in the present study was very high, and it would be of interest to study the effect of considerably lower dietary levels.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514000373
Acknowledgements
The present paper reports the results of contract research mainly funded by BioMar A/S under the leadership of senior scientist Kjell Måsøval. The Aquaculture Protein Centre (APC) at the NVH, a centre of excellence under the Norwegian Research Council (project no. 145949/120), was the main scientific partner of the experiment. The molecular part of the experiment was funded by the APC. The authors thank the senior researcher Gerd Marit Berge and technicians at the Nofima research station at Sunndalsøra, Norway, for skilful animal care and scientific follow-up and APC's technicians Ellen K. Hage, Elin C. Valen and Gunn C. Østby for their excellent technical assistance.
The authors' contributions are as follows: T. M. K. performed the molecular analysis and analysed the data and wrote the manuscript; I. B. was responsible for the biochemical analysis and manuscript revision, A. K. and G. T. participated in the microarray analyses and revised the manuscript; Å. K. designed and conducted the feeding trial and coordinated the research.
None of the authors has any conflicts of interest to declare.















