INTRODUCTION
Shigellosis is endemic throughout the world and is hyperendemic in developing countries. In the late 1990s, the annual number of shigellosis episodes was estimated at 163·2 million in developing countries (with around 1 million deaths) and 1·5 million in industrialized countries [Reference Kotloff1, Reference Voetsch2]. More recent estimates of the global burden of shigellosis report a similar extent of morbidity but a significantly lower mortality due to lower case-fatality rates [Reference Bardhan3]. Clinical manifestation of shigellosis can vary from moderate diarrhoea to severe dysentery. Children with dysentery from developing countries have an increased risk for persistent diarrhoea, nutritional faltering, and death [Reference Niyogi4–Reference Black7]. Four Shigella serogroups: S. dysenteriae (known to have 13 serotypes), S. flexneri (16 serotypes), S. boydii (20 serotypes), and S. sonnei (one serotype), are the aetiological agents of shigellosis. Shigella is also an important aetiological agent of diarrhoea in travellers and in soldiers deployed to endemic regions [Reference Niyogi4, Reference Hyams8]. Shigellae are highly contagious with infectious doses of as low as 10–100 viable bacterial cells [Reference DuPont9]. Transmission occurs via the faecal–oral route, usually from person-to-person contact or by intake of contaminated food or drinking water [Reference Niyogi4]. Significant fly-borne transmission of Shigella has also been documented [Reference Cohen10].
Despite the improved socioeconomic conditions, Israel has remained a highly endemic area for shigellosis [Reference Green11, Reference Ostroi, Anis and Green12]. It has been shown that children aged 1–4 years and soldiers serving in field units are at increased risk of developing shigellosis [Reference Green11, Reference Ostroi, Anis and Green12].
The present report provides an update on the burden and epidemiology of shigellosis in Israel, describes the recent cyclic pattern of occurrence of epidemics of shigellosis, and comments on and points out host- and agent-related characteristics of the disease. These data are important for designing intervention programmes to reduce the burden of the disease.
METHODS
Shigellosis is a notifiable disease in Israel. Reports on culture-proven cases of shigellosis are submitted by bacteriological laboratories throughout the country to the corresponding public health districts and then collated at the Department of Epidemiology of the Ministry of Health. This passive surveillance system which was established at the beginning of the 1950s provides long-term assessment of the trends in the incidence of the disease in Israel. However, it lacks information on the Shigella serogroup and serotype for each case and demographic data on the cases are incomplete [13].
An additional, active surveillance system for bacterial enteric diseases (shigellosis, salmonellosis, campylobacteriosis), based on sentinel community and hospital microbiological laboratories located throughout Israel, was established in 1997. Data on the isolation of the enteropathogens including Shigella at the sentinel laboratory, demographic information related to patients and characterization of isolates at the National Reference Centers of the Ministry of Health, are collated at the Israel Center for Disease Control (ICDC) (Fig. 1) The population served by the five community laboratories represents 31·0% of the total Israeli population and its structure is almost identical to that of the total Israeli population according to gender and age groups. There is a slight overrepresentation of the Israeli Arab subpopulation, 25·7% vs. 20.5% in the general population. Israeli Jews and Israeli Arabs are citizens of the State of Israel residing in Israel at the time of the study.

Fig. 1. Community sentinel laboratories included in the study and their location on the map of Israel. Clalit Health Maintenance Organization (HMO) laboratories in Afula (A) and Haifa (H) representing the North of the country; Clalit HMO laboratory of Soroka Medical Centre in Beer Sheva (S) serving the population in the South; Meuheded HMO laboratory in Jerusalem (M) and Maccabi HMO laboratory of Ramat Gan/Mahoz Dan (D) serving the population in the centre of Israel. The National Reference Centres of the Ministry of Health and the Israel Centre for Diseases Control are located in Jerusalem and Ramat Gan, respectively.
Isolation and identification of Shigella and serogrouping were performed at the sentinel clinical microbiological laboratories using similar routine microbiological procedures. Identification of Shigella serogroup was confirmed at the Shigella Reference Laboratory of the Israel Ministry of Health. This was followed by the definition of serotypes using monovalent antisera (Denka Seiken, Japan). Susceptibility to antimicrobial agents was determined in all S. flexneri and in a systematic sample of S. sonnei isolated during 2000–2008. This was done at the Shigella National Reference Laboratory of the Ministry of Health using the Kirby–Bauer disk diffusion method [Reference Bauer14], on Muller–Hinton agar (Difco, BD Bioscience, USA) following the guidelines of the Clinical and Laboratory Standards Institute [15]. Commercially manufactured discs (Oxoid, UK) containing six antimicrobial agents were used: 10 μg ampicillin (AMP), 25 μg trimethoprim-sulphamethoxazole (SXT), 30 μg nalidixic acid (NAL), 30 μg chloramphenicol (CHL), 30 μg ceftriaxone (CRO), and 30 μg tetracycline (TET). Multi-drug resistance (MDR) was defined as resistance to three or more antimicrobial agents.
A case of shigellosis was defined as a subject with a positive stool culture for Shigella spp. A case of shigellosis was counted only once when Shigella of the same serotype was isolated from stool specimens submitted during the acute and convalescent stages of the diarrhoeal episode (within 1 month after the first isolation).
For the computation of incidence rates, the sizes of the catchment populations of the different sentinel laboratories were calculated based on data received from the Central Bureau of Statistics and the Health Maintenance Organization (HMO) to which the laboratories belonged.
Adjusted incidence rates were calculated based on data from population and physician surveys and from HMO databases used to determine the gaps in the various surveillance steps and the reciprocal multipliers for calculation of the diarrhoeal disease burden in childhood in Israel [Reference Ziv16], and the approach previously described to determine the burden of shigellosis in Thailand and salmonellosis in the USA, respectively [Reference Voetsch2, Reference Chompook17]. The multipliers for each surveillance step were the inverse of the proportion of subjects responding positively [Reference Voetsch2]. The overall multiplier was the product of the multipliers for each surveillance step. Based on the previous report [Reference Ziv16] the proportion of subjects complying with the various surveillance steps were: 38·8% in the 0–17 years age group (we estimated only 30% for all age groups) who had a diarrhoeal disease and visited a physician; 24% were referred by the physician to provide a stool specimen (75% of whom provided a stool specimen); 70% sensitivity of the stool culture for detection of Shigella spp. [Reference Chompook17, Reference von Seidlein18]; and 100% reporting from sentinel laboratories to ICDC on Shigella isolates. The respective multipliers were: 3·3 (or 1:0·3), 4·2, 1·3, 1·4 and 1. The overall multiplier was 25·2.
We examined the extent of protection conferred by S. sonnei shigellosis contracted in one outbreak against the onset of homologous repeat disease during the outbreak occurring 2 years later. To this end, we determined in each new outbreak the attack rate of S. sonnei shigellosis in subjects who were reported as culture-proven cases of S. sonnei shigellosis in the previous outbreak (by cross-checking the identifiers of the culture-proven cases in the two outbreaks) and in the rest of the children for whom there was no report on S. sonnei shigellosis in the previous outbreak. We then calculated the relative risk of repeat disease for each outbreak and overall, and estimated the extent of protection conferred by homologous culture-proven disease according to the formula:
We restricted this analysis to children aged 0–4 years belonging to the catchment population of laboratory M in Jerusalem. As a rule, the denominators in the second outbreak included only children aged <4 years.
To exemplify in numbers for the outbreak in 2006: four repeat cases of S. sonnei from 405 S. sonnei cases identified in 2004 (attack rate 9·9/1000); 578 cases of S. sonnei shigellosis in 11 838 children for whom there was no report on S. sonnei shigellosis in 2004 (attack rate 48·8/1000). The relative risk and protective efficacy yielded by these figures were 0·2 [95% confidence interval (CI) 0·08–0·54] and 79·8% (95% CI 46·0–92·4), respectively.
Analysis of data was performed using SPSS v. 21 (IBM Corp., USA). In addition, WINPEPI Computer Programs for Epidemiologists [Reference Abramson19] was used to calculate incidence rates/100 000 Pearson's χ2 P values and 95% confidence intervals.
RESULTS
Figure 2 depicts data generated by the passive surveillance system showing that in the population of Israel the average incidence of culture proven-cases of shigellosis is around 70–100 cases/100 000 per year [13]. After being relatively stable until around 1974, the incidence of shigellosis gradually increased to a peak in 1985 associated with a large waterborne epidemic of S. sonnei shigellosis in the Haifa region [Reference Egoz20]. This was followed by an overall decline until the beginning of the 1990s and by a change in the pattern of disease occurrence later on characterized by peaks in the incidence of shigellosis occurring every 2–3 years (Fig. 2).

Fig. 2. Total cases and incidence rates of shigellosis in Israel based on passive reporting on culture-proven cases of disease by microbiological laboratories and physicians, 1951–2012 [Reference Ziv13].
The incidence rates of shigellosis for the overall population served by the five community sentinel laboratories between 1998 and 2012 followed a clear cyclic pattern and ranged from 89–178 to 25–83 cases/100 000 in the years of high and low incidence of shigellosis, respectively. Out of 30 337 Shigella isolates at the sentinel community laboratories 85·3% were S. sonnei and 11·3%, 2·5% and 0·7% were S. flexneri, S. boydii and S. dysenteriae, respectively. The incidence rates of S. sonnei shigellosis in the epidemic years were significantly higher than those in the consecutive non-epidemic years (Table 1). In the Jewish population, 93·7% (n = 24 808) of the new cases of shigellosis were S. sonnei shigellosis following the cyclic pattern of peaks of morbidity (Fig. 3). By contrast, in the Israeli Arab population, 50·3% of the new cases of shigellosis (n = 4084) were caused by S. flexneri, 34·4% by S. sonnei, 12·2% by S. boydii, and 3·0% by S. dysenteriae (Fig. 4).

Fig. 3 [colour online]. Incidence of shigellosis by Shigella serogroup in the Jewish population served by the sentinel laboratories.

Fig. 4 [colour online]. Incidence of shigellosis by Shigella serogroup in the Arab population served by the sentinel laboratories.
Table 1. Incidence rates and rate ratios of Shigella sonnei shigellosis in epidemic and non-epidemic years in the population served by the sentinel laboratories
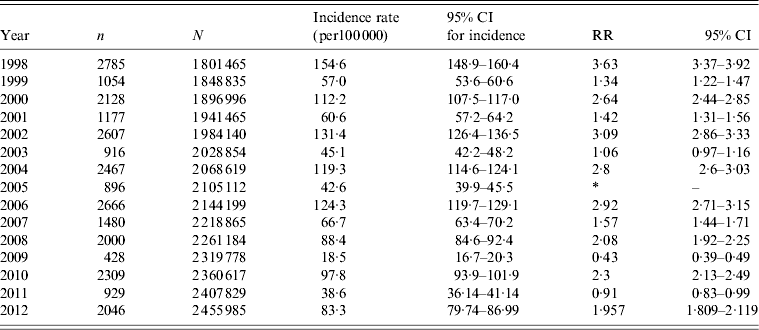
RR, Rate ratio; CI, confidence interval.
* Reference category = 2005.
Overall, the average annual incidence of shigellosis was 97/100 000 throughout the 15 years of surveillance. It was higher in the Jewish population compared to the Arab population (105/100 000 vs. 55/100 000 respectively; P < 0·001).
The age-specific incidence rates were the highest in the 0–4 years age group with values ranging from 569–939 to 139–496 cases/100 000 in the years of high and low incidence of shigellosis, respectively (Fig. 5). Throughout the 15 years of surveillance, the 0–4 years age group had the highest incidence rates of shigellosis in both the Jewish (an average of 721/100 000 mostly due to S. sonnei) and Arab (an average of 248/100 000) populations. Within the 0–4 years age group and in Jews, the lowest incidence was found in the first year of life and the highest in the third. However, for Arabs the highest incidence was in the second year followed by the first year of life. The incidence of shigellosis was significantly higher in Arabs (287/100 000) compared to Jews (165/100 000) (P < 0·001) in the first year of life while in all other age groups the incidence was significantly higher in Jewish children compared to Arab children.

Fig. 5 [colour online]. Incidence of shigellosis in the various age groups of the general population served by the sentinel laboratories.
In the 0–4 years age group, the female/male risk ratio was 0·88 and 0·81 for Jews and Arabs, respectively (P < 0·001 in both populations), and significantly >1 in the 15–24, 25–34, 35–44, 45–54 and 55–64 years age groups (Table 2).
Table 2. Average incidence of shigellosis for 1998–2012 in the population served by the sentinel laboratories by age, sex and ethnicity
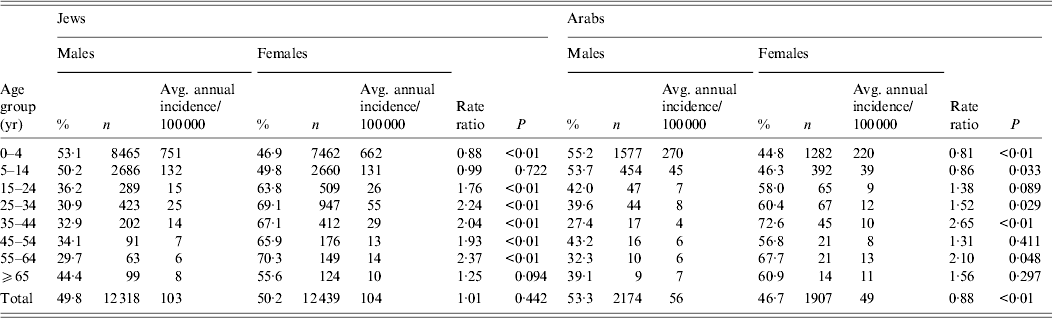
The adjusted average incidence of shigellosis in the community for the whole Israeli population was 2425/100 000 per year during 1998–2012 after using the multiplier of 25 to control for estimated gaps in the surveillance steps (see also Methods section).
Characteristics of S. sonnei shigellosis
The incidence of S. sonnei increased constantly from January to July with a significant drop in April and reached its peak in July. The earliest and steepest increase in incidence of S. sonnei in the epidemic years occurred in winter, in the M and D laboratories, representing the Jerusalem and Central districts (Fig. 1). This was usually followed by a significant increase in the number of isolates in laboratory S covering the South of Israel. The M and D laboratories serve the Jewish Orthodox religious neighbourhoods of Jerusalem and Bnei Brak, respectively, inhabited by families with a large number of children living in crowded conditions.
The average serotype-specific protective efficacy conferred by S. sonnei infection for a 2-year period was 81·8% (95% CI 69·9–89·0), derived from calculated protective efficacies of 87·3% (95% CI 60·6–95·9) in the 2002 outbreak, 95·4% (95% CI 67·4–99·4) in the 2004 outbreak, 79·8% (95% CI 46·0–92·4) in the 2006 outbreak and 51·3% (95% CI 46·0–92·4) in the 2008 outbreak.
After 8 years of almost 100% resistance of S. sonnei to trimethoprim/sulfamethoxazole, a significant decrease to 80% occurred, as in the case of resistance to tetracycline, which dropped from 80% in 2000 to 10–20% between 2002 and 2008 (Fig. 6). S. sonnei isolates showed a consistent resistance rate of 80% to ampicillin while that to nalidixic acid, chloranphenicol and ceftriaxone did not exceed 5% and 1%, respectively, since 2002 (Fig. 6).

Fig. 6 [colour online]. Resistance pattern of S. sonnei isolates (n = 3642) to six antimicrobial agents during 2000–2008.
Characteristics of S. flexneri shigellosis
The incidence of S. flexneri shigellosis showed a constant summer increase, from April to August, with its peak in July. Sixty-three percent of S. flexneri were isolated from Arab patients, mostly in the 0–4 years age group. S. flexneri 2a and S. flexneri 6 alternated as being the two most common serotypes during the 15 years of surveillance accounting for ∼71% of the isolates. S. flexneri 1b was the third most frequently isolated serotype. In 2010 an outbreak of S. flexneri 6 occurred in the Orthodox Jewish community in Jerusalem and its surroundings. Thirty-five percent (n = 416) of all S. flexneri 6 isolates were isolated in 2010.
The resistance rate of S. flexneri (n = 2173) to ampicillin, tetracycline, trimethoprim/sulfamethoxazole and chloramphenicol ranged mostly between 60% and 80% from 2000 to 2008 with some significant annual fluctuations (Fig. 7). There was a significant decrease in the resistance rate to trimethoprim/sulfamethoxazole which reached 40% in 2007 and 2008. The resistance rate to nalidixic acid and ceftriaxone did not exceed 5% and 1%, respectively.

Fig. 7 [colour online]. Resistance pattern of S. flexneri isolates (n = 2173) to six antimicrobial agents during 2000–2008.
Of S. sonnei and S. flexneri isolates 12·1% and 72·9%, respectively, were multidrug resistant (resistant to three or more antimicrobial agents).
DISCUSSION
The sentinel laboratory-based active surveillance network for shigellosis was established in 1998 as an add-on to the passive surveillance system of notifiable infectious diseases (including shigellosis) instituted in Israel at the beginning of the 1950s. It provides comprehensive information on demographic variables related to subjects from whom Shigella sp. was isolated and also the complete serological characterization of the Shigella isolates.
S. sonnei was responsible for more than 85% of the cases of shigellosis in Israel during 1998–2012. S. flexneri was the second most prevalent Shigella serogroup (11·3%). In 1993 S. sonnei and S. flexneri accounted for 77% and 19%, respectively, of Shigella isolates characterized at the Ministry of Health Shigella Reference laboratory [Reference Ostroi, Anis and Green12]. Interestingly, during 1998–2012, S. sonnei was identified in 93·7% of the new Shigella isolates in the Jewish population and in only 34·4% of the Shigella isolates in Israeli Arabs where S. flexneri was the leading serogroup accounting for 50·3% of the Shigella isolates. The increase in the predominance of the relative importance of S. sonnei compared to S. flexneri and the other Shigella serogroups (S. boydii, S. dysenteriae) is a trend characteristic to developed countries [Reference von Seidlein18, Reference Gupta21, Reference Lerman22], while S. flexneri remains the leading Shigella serogroup in developing countries or in subpopulations of lower socioeconomic level, living in rural areas and with lower levels of sanitation [Reference von Seidlein18, Reference Sack23, Reference Vinh24]. The serotypes S. flexneri 2a and S. flexneri 6 alternated as being the two most common serotypes during the 15 years of surveillance as also reported from many other countries [Reference Levine25] and supporting the inclusion of both serotypes (or respective O polysaccharides) in candidate multivalent Shigella vaccines [Reference Levine25].
S. sonnei was associated with the occurrence of country-wide epidemics of S. sonnei shigellosis in Israel every 2 years. The clearest cyclic pattern and the highest incidence of disease were found in the 0–4 years age group and in the catchment area of M and D laboratories, which provide microbiological laboratory services to the Jewish Orthodox religious neighbourhoods of Jerusalem and Bnei Brak, inhabited by families with a large number of children living in crowded conditions. A lower level of exposure of an infant compared to a toddler, to person-to-person, foodborne transmission and transmission by fomites of Shigella, and on the other hand, a potential protective level of S. sonnei antibodies of maternal origin could explain the lowest incidence of shigellosis in the first year of life. Toddlers, already mobile, are at a high risk of being in contact with faecal material while they are in transition from the stage of using diapers to the stage when they are completely trained to use the toilet for defecation. Inadequate hand-washing, diapering practices and a high toddler–toilet ratio have been associated with S. sonnei transmission in daycare centres or in households [Reference Khan26–Reference Mohle-Boetani30]. In addition, child-to-child transmission, caregivers who both prepare meals and change diapers [Reference Mohle-Boetani30] or just various fomites at the daycare centres or at home can serve as vehicles and augment the transmission of Shigella [Reference Shane29, Reference Mohle-Boetani30]. It has been shown that attendance at a daycare centre or a pre-school setting were the main risk factors for primary cases in affected households in an outbreak of S. sonnei shigellosis in similar Jewish Orthodox communities in New York [Reference Shane29, Reference Garrett31]. Conditions of crowding and a younger age of the primary case, strongly support secondary transmission in households augmenting the outbreak [Reference Garrett31].
Interestingly, in Israeli Arabs the highest incidence of shigellosis (mostly caused by S. flexneri) was in the second year of life followed by the first year, suggesting that significant exposure to Shigella occurs earlier after birth in Arab children compared to Jewish children.
Similar differences between Israeli Arabs and Jews were described for the age-specific incidence of hepatitis A, another faeco-orally transmitted disease [Reference Green32]. This finding, as well as the leading role of S. flexneri in the aetiology of shigellosis, can be related to lower environmental and socioeconomic conditions and living predominantly in a rural environment in Arab children compared to Jewish children, which may offer a wider range of modes of transmission of Shigella early in life.
The increase in the number of new cases of S. sonnei shigellosis usually started in the winter months, possibly enhanced by more intimate contact in households and daycare centres during the time spent in indoor activities and peaked in July after a consistent drop in April. April is usually the month of the Passover vacation which may break temporarily the chain of person-to-person propagation of S. sonnei when children are absent from daycare centres, kindergartens and schools. S. flexneri had a consistent summer increase and much lower number of isolates during the rest of the year suggesting potential involvement of multiple modes of transmission enhanced by the higher summer temperatures.
We assume that females in the 15–44 and 45–64 years age groups with 50–100% excess of shigellosis compared to males comprise mothers, grandmothers or caregivers who had a much higher risk of exposure to Shigella while taking care of sick children. Similar age- and sex-related patterns of infection have been reported previously from other laboratory-based surveillance networks [Reference Gupta21, Reference Rosenberg33]. Previous observational and experimental studies in humans reported findings on serotype-specific immunity following Shigella natural infection [Reference Lerman22, Reference Herrington34, Reference Ferreccio35]. In this study we were able to quantify the extent of protective efficacy conferred by naturally acquired S. sonnei shigellosis (around 80%) against the homologous Shigella serotype when epidemic exposure re-occurred 2 years later. We have shown that serum IgG anti-Shigella lipopolysaccharide (LPS) antibodies, which are elicited by natural infection, are strongly associated with protection against homologous disease [Reference Cohen36, Reference Cohen37]. We assume that there is an association between the cyclic peaks of morbidity due to S. sonnei and changes in the level of natural immunity to the organism acquired by the affected population. An outbreak of shigellosis occurring in children in the 0–4 years age group will be followed by an increase in the level of natural immunity to the homologous Shigella organism (S. sonnei) which will also provide the level of herd immunity sufficient to prevent the onset of a new epidemic. After 1 or 2 years, waning in the level of anti-LPS antibodies together with the intake of a new cohort of naive newborns will lead to a decrease in the level of heard immunity below a critical level. Under high and continuous risk of exposure to Shigella in children aged 0–4 years, living in crowded conditions, this will allow the renewal epidemic transmission of S. sonnei.
There were similarities and also differences between the antibiotic resistance patterns of the two leading Shigella serogroups in Israel during 2000–2008. Both S. sonnei and S. flexneri isolates showed high rates of resistance to commonly used antimicrobial agents such as ampicillin and trimethoprim/sulfamethoxazole. These resistance rates are similar to or higher than those reported in Israel in the mid-1990s [Reference Mates, Eyny and Philo38, Reference Vasilev39] and in other countries more recently [Reference Vrints40, Reference Shiferaw41]. On the other hand, both S. sonnei and S. flexneri displayed very low rates of resistance to quinolones (nalidixic acid) and third-generation cephalosporins (ceftriaxone) as also found in a series of studies conducted globally [Reference Vrints40–Reference Banga44] with the exception of some of the Asian countries where resistance to these antibiotics has significantly increased [Reference von Seidlein18, Reference Vinh24, Reference Banga44]. Resistance of S. sonnei to tetracycline fell markedly from 2000 to 2002 and remained low thereafter. Similar differences between S. sonnei and S. flexneri in percent of resistance to tetracycline and chloramphenicol found in our study were also detected in the FoodNet sites in the USA between 2000 and 2010 [Reference Shiferaw41].
The present study has some limitations. We are dealing with the tip of the iceberg in respect of the actual burden of shigellosis in the community. To have a culture-proven case of shigellosis means that the patient must seek medical care first, the physician must order a stool culture, a stool specimen must be obtained and submitted to the laboratory for culture and the laboratory has to isolate the organism. On the one hand, data on culture-proven cases in community laboratories could select for moderate to severe cases of shigellosis compared to those who would not visit a community clinic. On the other hand, we did not include Shigella isolates from hospitalized patients since we did not have a denominator for them to calculate incidence rates. In this case we could have lost the most severe cases of shigellosis requiring direct hospitalization. There might be also a differential utilization of medical services and health-seeking behaviour in respect to diarrhoeal diseases in the Jewish and Arab subpopulations possibly leading to a differential underestimation of the burden of shigellosis in Israeli Arabs. We determined an estimated multiplier of 25 to close the gaps in the surveillance steps and estimated the adjusted average incidence of shigellosis in the community for the whole Israeli population at 2425/100 000 per year during 1998–2012. Applying this figure to the population of Israel (8 012 400 inhabitants as of 31 March 2013) we reached the estimate of 194 300 outpatient cases of shigellosis per year. Assuming an additional 10% of inpatient cases, the approximate total average number of new cases of shigellosis per year will be 213 730 which represents an impressive burden especially when referring to the epidemic years.
With regard to the estimates of protection, it might be possible that the number of repeat S. sonnei shigellosis identified in a new outbreak in children who suffered from the disease in the first outbreak, 2 years previously, might be lower than expected thus overestimating the protective efficacy conferred by natural S. sonnei infection. This could be due to potential lower exposure since these children will be 2 years older (but still in the 0–4 years age group), to a lower chance that they and their parents will seek care, being already experienced with the diseases from the previous episode, or perhaps just having a milder repeat disease as a result of potential partial protection thus reducing the chance that they would be identified.
CONCLUSIONS
The sentinel laboratory-based surveillance network led to the identification of the cyclic occurrence of S. sonnei epidemics in Israel. The burden of shigellosis is high. Intervention programmes including promotion of personal hygiene with special emphasis on hand washing are needed to prevent the transmission of shigellae when the first clusters of disease occur. Immunization of children in the 1–4 years age group with an efficacious S. sonnei vaccine when such a vaccine is available should be considered as the means to interrupt the cycles of morbidity and significantly reduce the burden of shigellosis in Israel.
ACKNOWLEDGEMENTS
The study was supported in part by grant agreement no: 261472 STOPENTERICS from the European Union Seventh Framework Programme. We thank Rachel Japheth for laboratory support in characterization of Shigella and Nadia Pekurovski for assistance with data collection from the sentinel laboratories.
DECLARATION OF INTEREST
None.














