Salmonella spp. is a leading cause of foodborne illness in the USA [Reference Scallan1]. Transmission occurs by ingesting the bacteria through contaminated food or water or ingesting faeces from an infected person or animal [Reference Heymann2]. People infected with Salmonella develop a gastrointestinal illness (diarrhoea, fever, abdominal cramps) from 6 to 72 h after infection [Reference Heymann2]. This infection can spread into septicaemia and, in rare cases, to other body sites. Becoming infected with this bacterial illness can result in hospitalization and death [Reference Scallan1].
Although poultry and eggs are the traditional vehicles for foodborne salmonellosis, there have been contaminated products other than poultry or eggs that have caused Salmonella outbreaks [3–6]. Salmonella contamination of low-moisture food products is one example. Low-moisture food products identified in the past with Salmonella are nuts, seeds, cereal, peanut butter, chocolate and powdered milk [7, Reference Podolak, Enache and Stone8].
Nuts and seeds have been identified as sources of Salmonella outbreaks. Examples of contaminated nuts and seeds are hazelnuts, peanuts, pistachios, pecans, sunflower and sesame seeds [Reference Podolak, Enache and Stone8]. These foods may be contaminated with Salmonella due to the process of harvesting, mechanical processing, cross-contamination and storage [Reference Podolak, Enache and Stone8, 9]. These products may pose an increased risk to consumers because they are often consumed raw. Salmonella is a reportable disease that all local health departments in New York State (NYS) are mandated to investigate under the NYS sanitary code. All suspected cases are contacted and interviewed with a standard questionnaire that identifies symptom history, food and water sources of exposure, travel history, grocery store purchases, food eaten outside the home, occupation and living environment. The case interview is then entered into an electronic medical record that is submitted to the New York State Department of Health (NYSDOH).
In September 2011, an increase in salmonellosis cases above the Monroe County historical levels was observed. Monroe County, which has Rochester as its major city, has a population of about 745 000 and is located in western NYS with Lake Ontario along its northern border. Mapping software (ArcGIS® version 10; ESRI, USA) was used to geocode home residence address data. A new layer was developed based on these geocoded points and overlaid onto a map of Monroe County. The cases were initially stratified by serogroup because the serotypes were not identified from the local laboratory. A geographical pattern began to develop among the salmonellosis cases; the serogroup B residences tended to be on the western side of the county, while the serogroup D cases were frequently located on the eastern side of the county (see Fig. 1 for the final outbreak map).

Fig. 1 [colour online]. Map of Salmonella cases based on home address in Monroe County, New York from August to November, 2011.
Based on the GIS map, the Monroe County Department of Public Health (MCDPH) looked for other differences in the two clusters. A grocery store chain and restaurants were identified as different variables between the two clusters. The MCDPH and NYSDOH have a good working relationship with this grocery chain. The serogroup D cases were interviewed and asked if they used a shopper card for grocery purchases. If they used the shopper card, then they were asked to give written permission for the release of their shopper card purchase records to the MCDPH. NYSDOH contacted the grocery chain, and provided written permission for the release of the shopper card records. The grocery chain sent the records to the NYSDOH in the form of a spreadsheet. Analysis of the shopper card summary count results identified three food items as common purchases among serogroup D cases. These items were tomatoes, Hass avocados and Turkish pine nuts.
A hypothesis-generating questionnaire was developed specifically to enquire about these common products among the serogroup D cases. These cases were re-interviewed to learn about consumption and to see if they had any leftover product that could be collected and sent to the NYSDOH laboratory. Three people were identified that had leftover Turkish pine nuts. MCDPH coordinated with NYSDOH Western Region epidemiology staff to collect these products and submit for testing.
NYSDOH Wadsworth Center Laboratory confirms Salmonella-positive human cultures, identifies serotypes and conducts pulsed-field gel electrophoresis (PFGE) testing. Once PFGE results are identified, the pattern is uploaded to the PulseNet (the molecular subtyping network for foodborne disease surveillance) database [10]. If a pattern matches in the database, the NYSDOH notifies the local health department. Two separate serotypes and PFGE patterns were identified in the Monroe County clusters. Salmonella Enteritidis was identified as the serogroup D type and Salmonella Typhimurium was serogroup B. The leftover Turkish pine nuts cultured as S. Enteritidis and showed an indistinguishable PFGE pattern to the S. Enteritidis cases.
In the PFGE outbreak pattern of S. Enteritidis, a total of 23 cases matched. Illness onset dates were from 24 August to 19 November 2011 (Fig. 2). The median age of patients was 33 years (range 5–94 years); 68% of the cases were female. Twenty out of 23 people interviewed identified shopping at the same grocery store chain. Only one person was hospitalized, while two others visited the emergency department. Symptoms identified by the 23 cases included diarrhoea (100%), nausea (57%), vomiting (26%), headache (57%), fever (65%), and abdominal cramps (78%).
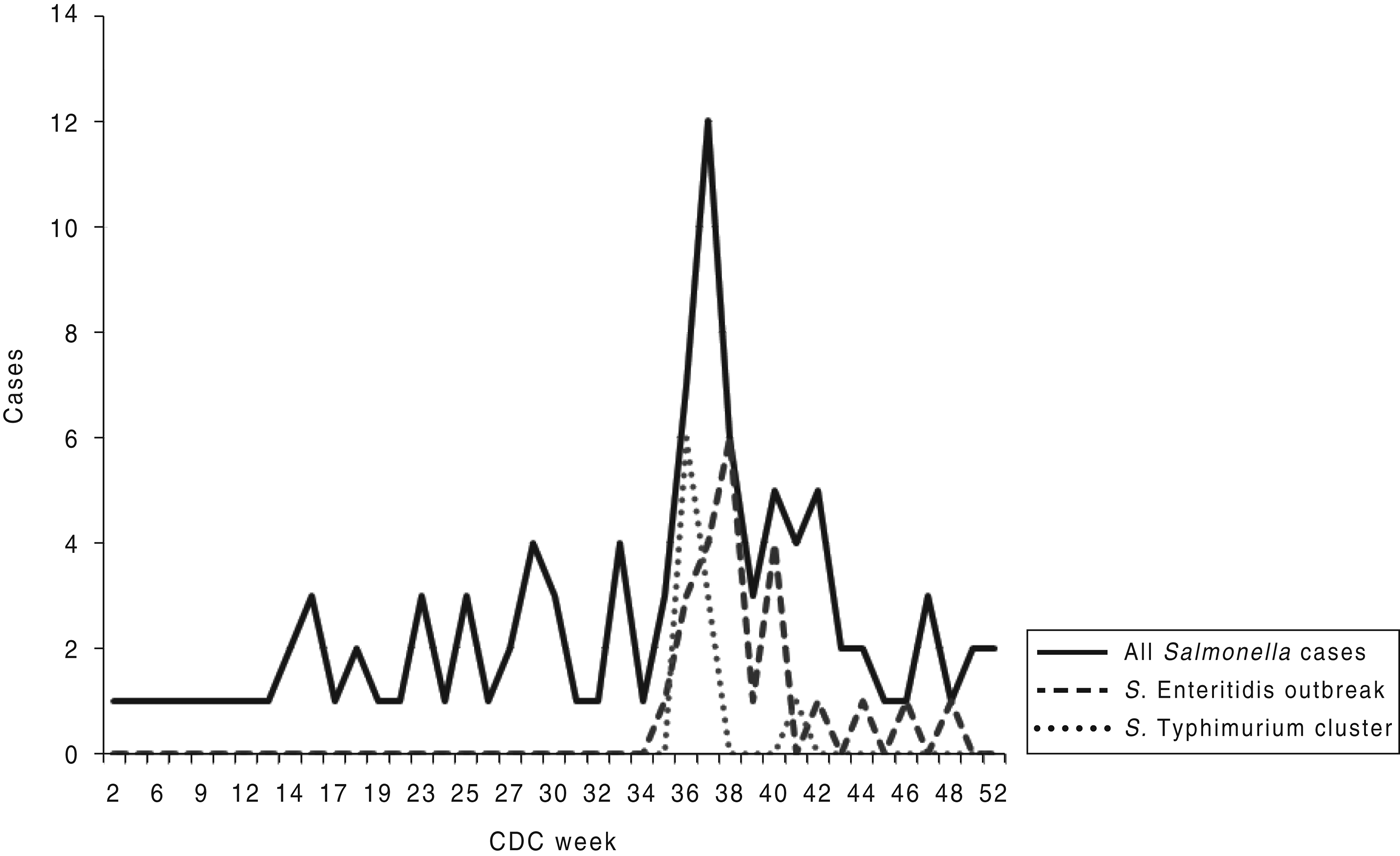
Fig. 2. Epi-curve for all Salmonella cases investigated in Monroe County in 2011 based on CDC reporting week.
Once the PFGE pattern was identified, it was used to identify additional cases throughout the state and country. A total of 43 people from five states were identified with the outbreak strain of S. Enteritidis [11]. On 26 October 2011, the grocery chain issued a voluntary recall of Turkish pine nuts from their stores. Subsequent recalls also occurred from the distributor.
To the authors’ knowledge, this is the first reported Salmonella outbreak associated with pine nuts in the literature. During this outbreak, the Turkish pine nuts were consumed raw or as an ingredient in prepared foods such as pesto sauce. These pine nuts were sold in bulk bins at the grocery store and were imported from Turkey. Although low-moisture food products do not have the proper sustenance to support bacterial growth, low numbers of salmonella can still cause illness. The problem with pine nuts, and nuts in general, is that they have a long shelf-life. This is depicted in the epi-curve in Figure 2. Moreover, pine nuts may be used as an ingredient in prepared foods and then frozen for consumption later, which occurred in some cases. The increasing number of significant Salmonella outbreaks associated with low-moisture foods emphasizes the need for proper environmental monitoring in processing areas and establishing procedures for controls and corrective actions [Reference Chen12].
For the S. Typhimurium cluster, which occurred during the same time-frame, a total of ten cases showed an indistinguishable PFGE pattern but did not result in a product being identified through the epidemiological investigation. Illness onset dates were from 28 August to 1 October 2011. The median age of patients was 31 years (range of 9–60 years); 50% of the cases were male. Four people were hospitalized. The GIS map (Fig. 1) details a clustering of restaurants where cases ate during the incubation period along a major traffic route in the county. A hypothesis based on the GIS map might indicate a common ingredient that was used and distributed to these restaurants. However, follow-up interviews with the restaurants did not identify a common distributor. A common food worker was also excluded in the investigation based on the number of restaurants identified. This cluster might have been due to a convenience food consumed by the cases at home that was not identified by case interviews. Whole genome sequencing might have been useful in determining more complete genetic polymorphisms for the cluster since the PFGE pattern identified was relatively common [Reference Hendrickson13].
Field epidemiology, GIS mapping, shopper card data, PFGE confirmation and product testing identified the cause of the S. Enteritidis outbreak. The use of GIS mapping in a communicable disease investigation should be considered routinely as a tool for epidemiologists. The authors did not use spatial scan statistics such as SatScan software, but this could have also provided an additional quantitative approach in conducting disease surveillance for detecting clusters of illness [Reference Odoi14]. Most cases of Salmonella that are investigated tend to be singly isolated and the source of infection is never confirmed. The cluster of S. Typhimurium that occurred during the same time-frame points to the difficult challenges for public health epidemiologists in identifying a source of exposure.
ACKNOWLEDGEMENTS
We thank Drew Sacheli, RN, MPA, Donna Hubbard, Chittadaphone Phouthavong, LPN, John Ricci, MS, and Stephanie Kelly, BS, for general support at the Monroe County Department of Public Health, Rochester, NY. We also acknowledge Christina Hidalgo, MPH, Glenda L. Smith, BS, Jillian Karr, MPH, and Kari Burzlaff, MPH, for epidemiology support from the New York State Department of Health Western Regional Office.
DECLARATION OF INTEREST
None.







