Introduction
The term lichen refers to mutualistic symbiotic organisms composed of a fungus which is intimately associated with a photosynthetic partner (algae or cyanobacteria or sometimes both) and resulting in an autotrophic form of life that uses the carbohydrates produced by the photosynthetic partner to live (Gargaud et al. Reference Gargaud, Amils, Cernicharo Quintanilla, Cleaves, Irvine, Pinti and Viso2011). This successful symbiosis comprises of about 17 500 species and dominates about 8% of the Earth's terrestrial surface (Brodo et al. Reference Brodo, Sharnoff and Sharnoff2001) although lichen diversity might be much higher (Lumbsch et al. Reference Lumbsch2011). Lichen symbioses involve more than 14 000 fungi (∼19% of all known fungal species, Kirk et al. Reference Kirk, Cannon, David and Stalpers2001) and about 100 species of green algae and cyanobacteria (Nash Reference Nash2008). Cyanobacteria are the oldest oxygenic organisms, having evolved 3500 to 2800 million years (Ma) ago (Olson Reference Olson2006) while algae are supposed to have appeared between 1000 and 1500 Ma ago (Hedges et al. Reference Hedges, Blair, Venturi and Shoe2004; Yoon et al. Reference Yoon, Hackett, Ciniglia, Pinto and Bhattacharya2004). Both events occurred long before the evolution of land plants (425–490 Ma, Sanderson Reference Sanderson2003) or lichen-forming fungi (about 500 Ma ago, Floudas et al. Reference Floudas2012). During the period in which cyanobacteria evolved (3500 to 2800 Ma ago), the terrestrial atmospheric structure was remarkably different from the current one with little or no free oxygen and accordingly no ozone layer to block shortwave UV radiation (UV-C and UV-B). Thus, unfiltered solar UV radiation (UVR) at much higher doses than at present reached the Earth's surface, including harmful UV-C (Kasting Reference Kasting1993; Castenholz & Garcia-Pichel Reference Castenholz, Garcia-Pichel, Whitton and Potts2002, Reference Castenholz, Garcia-Pichel, Whitton and Brian2012). Accordingly, cyanobacteria and other early organisms are supposed to have developed mechanisms towards UVR tolerance during that time. Regarding UV-A and UV-B effects on modern organisms, resistance capacities against radiation are more developed in organisms living in exposed habitats compared with those living in more protected environments (van de Poll et al. Reference Van de Poll, Hanelt, Hoyer, Buma and Breeman2002).
UVR has been a driving force for the evolution of life on Earth, acting both as a mutagen and as a selective agent (Rothschild & Cockell Reference Rothschild and Cockell1999; Rettberg & Rothschild Reference Rettberg, Rothschild, Horneck and Baumstark-Khan2002). The most lethal factor of the space environment, targeting DNA molecules and consequently having a deleterious effect on cells, is the full spectrum of solar UVR (Horneck Reference Horneck1999; Nicholson et al. Reference Nicholson, Schuerger and Setlow2005). Owing to the UV-shielding effect provided by the stratospheric ozone layer and other atmospheric components, the UV irradiance decreases from about 140 W m−2 above the atmosphere to about 32 W m−2 on the Earth's surface. The UVR reaching the Earth's surface is composed of UV-A (315–400 nm, 63 W m−2, 92.3% of total UVR), a small fraction of UV-B (280–320 nm, 5 W m−2, 7.4%) and a negligible percentage of UV-C (200–280 nm, <0.1 W m−2, <0.1%, according to Tevini & Häder Reference Tevini and Häder1985). Photosynthetic organisms use the light energy of the solar radiation to convert carbon dioxide and water into the sugars that will be used by the organism to fuel its biological functions. These organisms have not only evolved towards a maximal efficiency in the Sun's energy catchment in the ‘photosynthetically active’ range (PAR, 400–700 nm) where the solar energy output is maximum (∼43%, Nicholson et al. Reference Nicholson, Schuerger and Setlow2005) but also have developed different mechanisms to tolerate, counteract or avoid the UVR (essentially UV-A and a minor percentage of UV-B) that inevitably reaches their reaction centres (Larkum and Wood Reference Larkum and Wood1993). As many lichens dominate in areas with intense solar irradiation (Solhaug et al. Reference Solhaug, Gauslaa, Nybakken and Bilger2003) and endure harmful levels of this stressing factor (Sonesson et al. Reference Sonesson, Callaghan and Bjorn1995; Sass & Vass Reference Sass, Vass and Gareb1998), lichens have been intensively studied on their resistance to UVR. Several aspects were of special interest: the influence of UVR on lichen physiology in terms of secondary compounds synthesis induction (Solhaug & Gauslaa Reference Solhaug and Gauslaa1996, Reference Solhaug and Gauslaa2004; Buffoni Hall Reference Buffoni Hall2002; Solhaug et al. Reference Solhaug, Gauslaa, Nybakken and Bilger2003), biomass growth (Larsson et al. Reference Larsson, Vecerová, Cempírková, Solhaug and Gauslaa2009), adaptations for increased survivability (Nybakken et al. Reference Nybakken, Solhaug, Bilger and Gauslaa2004) as well as effects upon the photosynthesizing symbiont (Wynn-Williams et al. Reference Wynn-Williams, Holder and Edwards2000).
UVR (especially shortwave) imposes a dramatical threat on organisms (Horneck Reference Horneck1999; Nicholson et al. Reference Nicholson, Schuerger and Setlow2005) and causes indirect or direct damage on proteins, RNA and – most important – DNA. Indirect damage on DNA means that UVR induces the formation of reactive oxygen species (ROS), which react with DNA and impose oxidative stress (Horneck et al. Reference Horneck, Baumstark-Khan and Facius2006). Direct damage is caused by UVR-absorption of DNA itself (mostly around the absorption peak of DNA at 260 nm) and subsequent formation of cyclobutane pyrimidine dimers (CPDs) and 6,4 photoproducts (6,4-PPs). Such dimers alter DNA structure, hinder replication and are mutagenic (Britt Reference Britt1999). Some investigations have been done to assess how DNA is influenced by UVR in some lichen species (Buffoni Hall Reference Buffoni Hall2002; Rozema et al. Reference Rozema2002; Ünal & Uyanikgil Reference Ünal and Uyanikgil2011), but still little is known about how lichens repair UVR-induced damages. De Vera (Reference de Vera2005) demonstrated after 5, 10 and 20 kJ m−2 of UV-C254nm irradiation a dose-correlated formation of photoproducts in Xanthoria elegans. They were detected in the isolated photobiont (up to 90 photoproducts per 104 bp) and in intact thalli (up to 9 photoproducts per 104 bp) which are comprised of photobiont cells to ∼10% thallus dry weight (TDW). In contrast, the isolated mycobiont and mycobiont-formed tissues as apothecia did not form photoproducts under the respective UV-C doses. However, UVR is discussed to harm the photosynthetic apparatus by producing ROS that degrade the D1/D2 complex which is a central element of the photosystem II (PSII) (Jansen et al. Reference Jansen, Mattoo and Edelman1999).
Long before space exposure experiments with lichens, many lichen species were found to be extraordinarily resistant to temperature fluctuations and to desiccation stress (Lange Reference Lange1953) and their physiological adaptations to harsh environments were studied thoroughly; from the coldest polar areas to the deserts with the highest temperatures. In Antarctic lichens, gas exchange was measured down to about −20 °C and positive net photosynthesis take place even at −17 °C (Kappen et al. Reference Kappen, Schroeter, Scheidegger, Sommerkorn and Hestmark1996), while extended desert areas are covered by lichen vegetation in regions where the average annual precipitation is lower than 13 mm (Lange et al. Reference Lange, Green, Meyer and Zellner2007). Owing to their extremotolerant character, they were considered as suitable candidates for astrobiological exposure experiments in Low Earth Orbit (LEO, unshielded solar UVR (UV>170 nm), cosmic radiation, temperature fluctuations from −23 °C to +60 °C and vacuum of ∼10−6 Pa), which cause extreme desiccation, thermal stress as well as molecular and cellular damages by cosmic and solar radiation. The main aim of such experiments was to assess the survival capacity of lichens towards these conditions (Sancho et al. Reference Sancho, de la Torre, Horneck, Ascaso, de los Ríos, Pintado, Wierzchos and Schuster2007, Reference Sancho, de la Torre and Pintado2008).
The first astrobiology experiments that involved the exposure of lichens to space were carried out in three consecutive space missions: Lichens on the satellite FOTON M-2 in 2005 (Sancho et al. Reference Sancho, de la Torre, Horneck, Ascaso, de los Ríos, Pintado, Wierzchos and Schuster2007), Lithopanspermia on FOTON M-3 in 2007 (de la Torre et al. Reference de la Torre2010; Sánchez et al. Reference Sánchez, de la Torre, Sancho, Mateo-Martí, Martínez-Frías and Horneck2010; Raggio et al. Reference Raggio, Pintado, Ascaso, de la Torre, de los Ríos, Wierzchos, Horneck and Sancho2011) and LIFE on EXPOSE-E/EuTEF at the International Space Station (ISS) in 2008 (Onofri et al. Reference Onofri, de la Torre, de Vera, Ott, Zucconi, Selbmann, Scalzi, Venkateswaran, Rabbow, Sánchez Iñigo and Horneck2012; Scalzi et al. Reference Scalzi, Selbmann, Zucconi, Rabbow, Horneck, Albertano and Onofri2012). The first two experiments (that lasted approximately 15 and 10 days, respectively) demonstrated the high resistance and survival capacity of the species included, showing minimal changes in the vitality and a stable ultrastructure of the samples (Sancho et al. Reference Sancho, de la Torre, Horneck, Ascaso, de los Ríos, Pintado, Wierzchos and Schuster2007; de la Torre et al. Reference de la Torre2010). The third experiment with a duration of 1.5 years revealed lower rates of survivability (Onofri et al. Reference Onofri, de la Torre, de Vera, Ott, Zucconi, Selbmann, Scalzi, Venkateswaran, Rabbow, Sánchez Iñigo and Horneck2012). After the positive results of post-flight viability obtained in the previously described experiments, lichens were considered among the most resistant terrestrial organisms (Sancho et al. Reference Sancho, de la Torre and Pintado2008; de los Ríos et al. Reference de los Ríos, Ascaso, Wierzchos, Sancho, Green, Seckbach and Grube2010) and proposed to be ideal candidates for astrobiological research, focusing on their potential for intense studies about life's limits and to test the Lithopanspermia hypothesis on its plausibility (Sancho et al. Reference Sancho, de la Torre and Pintado2008; de la Torre et al. Reference de la Torre2010). Once the extremotolerant character of lichens was demonstrated, a systematical approach to explain the specific mechanisms of lichen resistance is required. One approach is to separately assess the influence of each space parameter on the physiology of both lichen symbionts. The high rates of survival rates after space exposure aroused interest in the unexpectedly high resistance of lichens towards UV-C (a stressor not existing on Earth) aroused the interest of the scientific community. It is now in focus of recent research to understand how these organisms are influenced by UV-C. The work done by de Vera & Ott (Reference de Vera, Ott, Seckbach and Grube2010b) was the first to individually analyse the single effect of artificial UV-C doses (from 2.1 J m−2 to 201.6 J m−2) on the resistance and viability of different lichen species from different habitats, detecting differences in the degree of viability that depend on the environmental conditions of the respective habitat. Organisms from areas with intense sun exposure and thus high UVR insolation have shown a greater resistance towards the space environment (de Vera and Ott Reference de Vera, Ott, Seckbach and Grube2010b). This first attempt to assess the effect of UV-C on lichens confirmed previous results: the bionts from the more naturally exposed to UVR lichen species Buellia frigida and X. elegans revealed a minor decrease in viability after the different UV-C exposures, neither while desiccated nor while wet. On the contrary, the lichen Peltigera aphthosa – more adapted to shady conditions – was severely affected by UV-C even at the lowest doses.
Desiccation tolerance mechanisms of Trebouxia sp. has been intensely studied (Gasulla Reference Gasulla2009), while just a little is known about their UVR tolerance mechanisms. In the present study we focus on the effects of UV-C radiation upon Trebouxia sp. (phylum Chlorophyta, class Trebouxiophyceae), which is the photobiont of two previously space-tested lichen species: Rhizocarpon geographicum and Circinaria gyrosa. R. geographicum was tested in the space exposure experiments Lichens, Lithopanspermia and LIFE as well as C.gyrosa in Lithopanspermia (references above). Owing to its remarkable ability in resisting space conditions, C. gyrosa was also used in a series of Mars simulation experiments that revealed a high survivability to short periods (Sánchez et al. Reference Sánchez, Mateo-Martí, Raggio, Meeßen, Martínez-Frías, Sancho, Ott and de la Torre2012). Based on such extremotolerance, C. gyrosa will be exposed to LEO parameters and simulated Mars conditions for a period of 15–18 months during the BIOMEX experiment (ESA call ILSRA-AO 2009) to be launched in April 2014 and exposed to the EXPOSE-R2 facility on board the ISS (de Vera et al. Reference de Vera, Boettger, de la Torre, Sánchez, Grunow, Schmitz, Lange, Hübers, Jaumann and Spohn2012).
The Trebouxia-photobiont was investigated when integrated in both lichen thalli and the astrobiological implications of the present results are discussed. We paid deeper attention to the damage on the PSII (Teramura & Sullivan Reference Teramura and Sullivan1994), the associated decrease on its photosynthetic efficiency and performance (Bornman Reference Bornman1989; Strid et al. Reference Strid, Chow and Anderson1990) as well as to the UV-C induced accumulation and/or degradation of the photosynthetic pigments chlorophyll a (chl a) and b (chl b) and two major accessory pigments with UVR screening properties: β-carotene and lutein. Chl a and chl b are crucial in photosynthesis and may indicate the state of the photosynthetic process in UV-C exposed Trebouxia sp. photobionts.
In lichens, UVR resistance is conferred by screening compounds that are a heterogeneous group of secondary lichen compounds (SLCs) with varying biosynthetic pathways (Huneck & Yoshimura Reference Huneck and Yoshimura1996) and whose synthesis is induced by UVR (Buffoni Hall Reference Buffoni Hall2002; Solhaug et al. Reference Solhaug, Gauslaa, Nybakken and Bilger2003). In lichens with a green algae as photobiont, polyphenolic compounds such as usnic acid and parietin are the most frequent UVR-screening compounds. Scytonemin is the most abundant in cyanobacteria-containing lichens (Cockell & Knowland Reference Cockell and Knowland1999). UVR-screening compounds represent a ubiquitous and effective method to reduce irradiation damage (Cockell & Knowland Reference Cockell and Knowland1999) that has been also proposed as a possible survival strategy in extraterrestrial habitats exposed to UV-C (Wynn-Williams et al. Reference Wynn-Williams, Edwards, Newton and Holder2002a).
The lichen thalli were subjected to increasing doses of UV-C in a range from 2.5×106 to 7.2×107 J m−2 under four different experimental conditions. The main objectives of this study were (1) to assess the influence of UV-C radiation as a single stressor upon Trebouxia sp., (2) to confirm the role of the metabolic state (active or inactive) of the thalli in terms of damage avoidance capability, (3) to study the protective role of pigments/cortex against UV-C radiation and (4) to deepen the understanding of the specific role of UVR resistance in the overall resistance capacity of the lichen symbiosis.
Materials and methods
Biological samples
Epilithic lichen species – R. geographicum
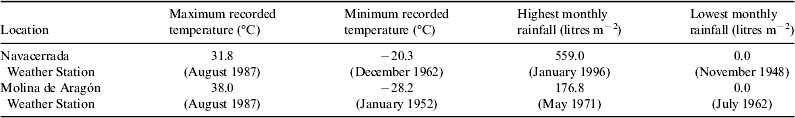
This crustose epilithic lichen (Fig. 1, A1–A3) is a cosmopolitan colonizer of alpine and polar regions (Feuerer, Reference Feuerer1991) that colonizes rocks. Our sampling site was the Navacerrada mountain pass, 40°46.9′N, 3°59.9W, 1938 m a.s.l. (Madrid, Spain). This area is covered by snow during long periods of winter but receives high levels of insolation and temperature during the summer season. The data recorded between 1946 and 2013 at the Navacerrada weather station show severe seasonal changes of temperature and precipitations (Table 1). Lichen-colonized rocks were collected considering three exclusive premises: homogeneous distribution of lichen on the granite rock surface, high level of colonization and flat surface of the rock.

Fig. 1. Different aspects of Rhizocarpon geographicum and Circinaria gyrosa: Two representative thalli of R. geographicum in the mountains of Navacerrada (Madrid, Spain) (A1) and of C. gyrosa in the steppic highlands of Zaorejas (Guadalajara, Spain) (B1); two light microscope pictures of R. geographicum and C. gyrosa (A2 and B2 respectively) displaying the internal stratification and distribution of both symbionts and two Scanning Electron Microscope (SEM) pictures showing the ultrastructural features of the internal organization of R. geographicum and C. gyrosa (A3 and B3 respectively).
Table 1. Some extreme meteorological parameters recorded between 1946 and 2013 in the Navacerrada weather station and between 1949 and 2013 in the Molina de Aragón weather station (adapted from Spanish Metereological Agency – AEMET, http://www.aemet.es)
Vagrant lichen species – C. gyrosa (nom. provis)
C. gyrosa (Fig. 1, B1–B3) has been recently renamed from Aspicilia fruticulosa (Sohrabi Reference Sohrabi2012) and is characterized by a dichotomous branched, coralloid thallus and a compact internal structure (Sancho et al. Reference Sancho, Schroeter and del Prado2000). Owing to its vagrant character, it grows detached from the substrate, having the possibility to be displaced freely by environmental factors. This type of living form is found in continental arid areas of Middle Asia, Eurasia, North America and Northern Africa (de la Torre et al. Reference de la Torre2010). The samples used in the present study were collected on clayey soil of the steppic highlands of Guadalajara (Central Spain high basins, Zaorejas, 40°44.691′N, 02°11.109′W, 1293 m a.s.l.). In this extreme environment, drastic diurnal and seasonal variations in terms of temperature, solar insolation and water availability occur. Some meteorological data (Table 1) registered between 1949 and 2011 at the Molina de Aragón weather station (40°50′40″N, 01°53′7″W, 1056 m a.s.l.) show maximum seasonal differences of more than 60 °C and a monthly rainfall range from 180 to 0 litres m−2 between the dry and the wet season.
Photobiont of both lichens – Trebouxia sp
Trebouxia is a genus of green algae (phylum Chlorophyta). Its relative abundance as photosynthetic partner is estimated to range from 20% (Rambold & Triebel Reference Rambold and Triebel1992) to 50% (Gärtner Reference Gärtner and Reisser1992) in all lichen species and up to 80% in the group of the green algae bearing lichens (Henssen & Jahns Reference Henssen and Jahns1974). The specimens of this genus live more or less exclusively lichenized and were rarely reported to be found free-living (Tschermak-Woess Reference Tschermak-Woess1978, Reference Tschermak-Woess1989; Bubrick et al. Reference Bubrick, Galun and Frensdorff1984; Ahmadjian Reference Ahmadjian and Reisser1992). In the thallus, they are restricted to the photosynthetically active algal layer, while the fungus contributes to about 90% of the lichen biomass. Trebouxia sp. is a coccoid green algae with a spherical to elliptic shape and thin walls (Fig. 2). No sexual reproduction has been observed in the symbiotic state (Friedl Reference Friedl1995) although genetic recombination was proposed (Kroken & Taylor Reference Kroken and Taylor2000). During the study described here, these algae were part of the lichen symbiosis and not isolated, as our main interest was to study the different response of the photobiont in its symbiotic state.

Fig. 2. Treboxia sp., the photobiont of the lichen species R. geographicum and C. gyrosa. Exemplified by photobiont cells isolated from C. gyrosa.
Sample preparation
Collection and processing of lichen samples
Collection and rock edition: R. geographicum colonized rocks were collected as a whole, while C. gyrosa samples were picked up individually from the substrate. Both were collected at their previously described sampling sites in a date close to the summer solstice (when the solar irradiation is maximum) to avoid seasonal vitality variations and the samples were frozen at −20 °C until further use. Owing to the epilithic and crustose character of R. geographicum, the lichen-bearing rocks were cut into cubes of 12 mm on side and 30 mm of height with a water-cooled diamond disc cutter, dried afterwards and kept at room temperature under dark conditions. The thalli of C. gyrosa were considered as single test samples and not processed.
Cortex removal of R. geographicum : Its high resistance towards UVR is supposed to be mainly provided by the cortex, while its removal leads to a short-term decrease of photosynthetic activity that is recuperated within a few days (de la Torre Noetzel Reference de la Torre Noetzel2002; de la Torre et al. Reference de la Torre Noetzel, Sancho, Pintado, Rettberg, Rabbow, Panitz, Deutschmann, Reina and Horneck2007a, Reference de la Torre, Garcia-Sancho, Horneck, Cockell and Horneckb). To investigate the protective role of the cortex against UV-C, the effect of its removal was assessed in half of the R. geographicum samples, taking it away carefully with a scalpel and leaving the algal layer directly exposed to UV-C.
Acetone treatment of C. gyrosa : To test the potential protective role of the sun-screening pigments on the photobiont, half of the total number of C. gyrosa samples were subdued to secondary lichen product extraction by four subsequent rinsings (5 minutes each) in 100% acetone (as described in Solhaug & Gauslaa Reference Solhaug and Gauslaa1996).
Revitalization
Before and after the irradiation experiment, all samples were subdued to a revitalization procedure similar to that performed in the previous space experiments Lichens (Sancho et al. Reference Sancho, de la Torre and Pintado2008), Lithopanspermia (de la Torre et al. Reference de la Torre2010) and LIFE (Onofri et al. Reference Onofri, de la Torre, de Vera, Ott, Zucconi, Selbmann, Scalzi, Venkateswaran, Rabbow, Sánchez Iñigo and Horneck2012). The samples were kept in a climatic chamber for 72 hours under controlled conditions of temperature (10 °C), PAR (day/night cycle of 12 hours/12 hours using a PAL mercury lamp with 100 μmol m−2 s−1 photosynthetic photon flux density (PPFD) and hydration (by spraying the samples till complete soaking once a day with mineral water). During revitalization, three measurements of the quantum yield (QY) efficiency of the PSII were performed on all samples (see subsubsection ‘Pre- and post-irradiation photosynthetic performance of the PSII’). After the last measurement, the revitalization process was stopped and the samples were air-dried at room temperature for 72 hours.
UV-C irradiation tests
To perform the irradiation tests with rising doses of UV-C, we used the BS-03 irradiation chamber (Dr. Gröbel UV-Elektronik GmbH, Germany) equipped with ten TUV 15W G15 T8 (Philips) low-pressure mercury tubes that emit short-wave polychromatic UV-C radiation in the spectral range comprised between 200 and 280 nm with a peak at 253.7 nm. The irradiation chamber was coupled with an external dose controller in the respective spectral range. The irradiation tests comprised the application of 23 doses of UV-C ranging from 2.5×106 J m−2 to a maximal dose of 7.2×107 J m−2 (refer to Table 2). The maximum dose is equivalent to an exposure of approximately 67 days (66.6±10.5 days) in LEO, as calculated by integration of environmental UV>170 nm data registered during BIOPAN I, II and III space flights (Horneck et al. Reference Horneck, Rettberg, Reitz, Wehner, Eschweiler, Strauch, Panitz, Starke and Baumstark-Khan2001). Four groups of three replicates were subjected to each irradiation dose:
-
(1) intact samples, dry (R. geographicum & C. gyrosa);
-
(2) intact samples, wet (R. geographicum & C. gyrosa);
-
(3) samples without cortex, dry (R. geographicum)/acetone rinsed, dry (C. gyrosa); and
-
(4) samples without cortex, wet (R. geographicum)/acetone rinsed, wet (C. gyrosa).
Table 2. Doses of UV-C radiation employed in the irradiation tests

After revitalization, the dry samples were directly placed in the irradiation chamber. However, the wet (and consequently metabolically active) samples were previously subjected to a maximum-hydration procedure spraying them with mineral water repeatedly for 20 minutes. During the whole irradiation period, the samples were kept hydrated by attaching mineral water-soaked filter paper to the base and sides of R. geographicum and to the base of C. gyrosa (refer to Fig. 3). The dose controller automatically stopped UV-C irradiation as soon as the respective doses were achieved.

Fig. 3. Irradiation chamber with the samples in the center of the irradiation area and the sensor located close to them. In the left front thalli of C. gyrosa are located on water soaked paper pads, in the right front there are cutted stones with crustose thalli of R. geographicum wetted by adjacent paper strips. The dry samples are depicted on a plastic tray in the center with six samples each of C. gyrosa (center left) and R. geographicum (center right).
Vitality assessment
Pre- and post-irradiation photosynthetic performance of the PSII
Before and after irradiation, the activity of the PSII of each sample was analysed three times by using a Mini-PAM fluorometer (Heinz Walz GmbH) at 24, 48 and 72 hours of the pre- and post-irradiation revitalization procedure (according to Sancho et al. Reference Sancho, de la Torre, Horneck, Ascaso, de los Ríos, Pintado, Wierzchos and Schuster2007). Optimum QY values were obtained after 20 minutes of dark adaptation from the equation: Fv/Fm=(Fm – Fo)/Fm with Fv=variable fluorescence yield, Fm=maximal fluorescence yield and Fo=minimal fluorescence yield (Schreiber et al. Reference Schreiber, Bilger and Neubauer1994). The comparison of pre- and post-irradiation QY measurements allowed determining the impact of UV-C on the photosynthetic performance of both lichen species for all four experimental conditions.
Pigments extraction and quantification
Pigment analyses were performed to assess the influence of UV-C radiation on the content and degradation of photosynthetic pigments – chlorophylls and carotenoids –. The content of the main antenna pigments chl a and chl b and two major accessory pigments, lutein and β-carotene was quantified. For each of the four experimental approaches and after the 72 hours revitalization period and subsequent PSII assessment, one sample was selected from six representative doses (the lowest of 2.5×106; an intermediate of 7.5×106; and the four highest of 1.2×107; 2.4×107; 4.8×107 and 7.2×107 J m−2, Table 2). A high performance liquid chromatography system (HPLC, Thermoquest IBM 360PL with SCM1000 degasser, P4000 quaternary pump, AS 3000 autosampler and a UV6000 PDA detector) was used to identify and quantify these pigments by comparison with standards.
Before pigment extraction the selected thalli were cleaned and lyophilized. All samples were weighed after the lyophilization to reference the amount of isolated pigment with its corresponding dry biomass. In the case of R. geographicum, the thalli were scratched from the rock to give realistic biomass weight. The samples were resuspended in 2 ml methanol for 48 hours at 4 °C and the supernatant methanol was filtered and transferred to HPLC vials. A Hipersil Gold reverse-phase column (Thermo Scientific, RP-18 250×4 mm) with a pore size of 5 μm was used. The sample injection volume was 100 μl with two solvents: (a) ethyl acetate and (b) acetonitrile:water 9 : 1 (v/v). In the mobile phase a linear gradient was employed with a flow gradient of 1.00 ml min−1 for 30 minutes, as described by Young et al. (Reference Young, Phillip, Savill and Pessaraki1997). The pigments were detected by an UV detector at 450 nm. Afterwards the samples were identified and integrated with known standards of chl a, chl b, β-carotene and lutein.
Statistical analysis
Two sets of statistical analysis were carried out with the results of PSII activity obtained from the overall experimental approaches performed (R. geographicum with/without cortex, dry/wet and C. gyrosa non-rinsed/rinsed, dry/wet) by one-way analysis of variance (ANOVA) with a significance level of p⩽0.05. In the first one, the degree of influence of each single UV-C dose applied on the post-irradiated means in relation with the pre-exposure measurements was assessed. In the second set of ANOVA tests, the whole set of pre-exposure PSII means was compared with the set of all post-irradiation means. Results will only be described as ‘significant’ if the ANOVA tests gave a significant difference.
Results
PSII performance before and after UV-C irradiation series
‘Rhizocarpon geographicum’
Intact samples in the dry and thus inactive metabolic state were not affected by the irradiation up to the highest applied dose of 7.2×107 J m−2 UV-C254nm. The QY was about 0.7 before and after exposure and did not change significantly with rising doses of UV-C (Fig. 4A). In the dry state, samples are supposed to be anabiotic and therefore prepared to face harsh conditions. Intact samples that were irradiated under wet conditions and were thus metabolically active showed a significant decrease in PSII performance with increasing doses of UV-C. While pre-exposure QY was comparable to the dry state (∼0.7), a continuous decrease to ∼0.3 at the highest dose was observed (Fig. 4B), indicating a harmful effect of UV-C on the activity of the PSII when the photobiont was physiologically active. The protective role of the cortex was not as important as the hydration status: if the lichen cortex is removed, dry thallus samples show an insignificant decrease of PSII activity of ∼0.05 after irradiation with higher doses of UV-C (Fig. 4C). Under wet, physiologically active exposure conditions the drop of PSII activity is significant compared with the wet but intact thallus samples. At the two highest doses of 4.8 and 7.2×107 J m−2 the QY dropped to zero (Fig. 4D). As these results are similar to the ones obtained with the intact thalli, it cannot be clearly concluded that the cortex prevents UV-C damage in the photosynthetic system of the lichen photobionts. The PSII activity is not or insignificantly decreased when R. geographicum is subjected to rising UV-C doses in its anhydrobiotic/anabiotic state. In contrast, the PSII activity strongly decreases under wet physiologically active exposure conditions demonstrating a negative correlation between UV-C dose and PSII activity.

Fig. 4. Photosystem II (PSII) activity of Rhizocarpon geographicum's photobiont (Trebouxia sp.) after each of the 23 UV-C irradiation doses and under the four different experimental conditions. Mean value±standard deviation given in each figure.
‘Circinaria gyrosa’
The samples under dry anabiotic conditions showed a constant pattern in terms of PSII activity before and after irradiation. Exposure to increasing doses of UV-C had no effect on the post-irradiation PSII performance, irrespective of whether the thalli were tested unaltered or rinsed with acetone before (compare Fig. 5A–C). All post-exposure measurements were comparable to the pre-exposure measurements that ranged between 0.6 and 0.75 QY. This unaffectedness was surprising taking into account the high UV-C doses during exposure. Wet and physiologically active samples showed a decreasing pattern demonstrating again that the photobiont is much more vulnerable while being wet. The PSII activity decreased with increasing UV-C doses. At a maximum dose of 7.2×107 J m−2, a significant decrease of the PSII activity to 0.4–0.45 QY in both intact and acetone-rinsed thallus samples was registered (Fig. 5B and D).

Fig. 5. Photosystem II (PSII) activity of Circinaria gyrosa's photobiont (Trebouxia sp.) after each of the 23 UV-C irradiation doses and under the four different experimental conditions. Mean value±standard deviation given in each figure.
Comparison of both lichen photobionts under the different experimental conditions
In terms of PSII activity, intact and dry samples of the two lichen species showed the smallest differences in the PSII activity before and after irradiation at all applied UV-C doses (Figs. 4A and 5A). The average pre-exposure QY is ∼0.7 and did not change significantly during both irradiation series. The results obtained with the intact and wet samples (Figs. 4B and 5B) were different. With R. geographicum, the post-irradiation QY values remained close to the pre-irradiation ones up to a dose of 4.5×106 J m−2, but showed a general tendency to decrease with higher doses, reaching ∼0.3 QY at the highest UV-C dose. With C. gyrosa, a slight decrease in photosynthetic performance was detected even at lower doses (compare Figs. 4B and 5B). However, in C. gyrosa the decreasing effect on PSII activity at doses above 9.5×106 J m−2 is less pronounced compared with R. geographicum, resulting in a lower loss of photosynthetic performance. The dry samples of R. geographicum with removed cortex as well as the dry acetone-rinsed samples of C. gyrosa showed no significant differences, neither if they are compared with the respective intact lichen samples nor to each other. The wet samples of R. geographicum with removed cortex as well as the wet acetone-rinsed samples of C. gyrosa showed no significant differences compared with their respective intact samples (compare Fig. 4B–D and Fig. 5B–D), except the completely reduced QY of R. geographicum at the maximum UV-C doses of 4.8 and 7.2×107 J m−2 (Fig. 4D, as mentioned above).
Analysing the PSII performance before and after the UV-C irradiation, several results became clear: (i) the removal of the cortex of R. geographicum led to a slight, but insignificant loss of PSII activity in the UV-C exposed algal layer. (ii) The acetone-rinsing of thalli of C. gyrosa, which was performed to extract putative screening lichen compounds, did not evoke any differences in the PSII activity compared with the non-rinsed thallus samples. (iii) In both lichen photobionts, the PSII activity of the thallus samples was significantly reduced by UV-C when wet. When metabolically active, the PSII activity is inversely proportional to the dose of applied UV-C.
Recovery of the PSII performance after irradiation with selected doses of UV-C
After UV-C exposure, all samples were revitalized for 24, 48, and 72 hours and their PSII activity was determined to assess its putative recovery. The results obtained after three representative doses (lowest: 2.5×106 J m−2; intermediate: 2.4×107 J m−2; highest: 7.2×107 J m−2) will be described below.
‘Rhizocarpon geographicum’
After applying the lowest UV-C dose (Fig. 6A), dry samples (intact and with removed cortex) showed a slight reduction of ∼0.05 QY after 24 hours which increased back to the initial pre-exposure values within the 72 hours period. Compared with pre-exposure values, both wet intact thalli and wet cortex-removed samples also showed a slight decrease of ∼0.05 QY that did not recover within the 72 hours period. After the medium dose (Fig. 6B) the PSII activity of dry intact samples decreased from 0.7 to 0.5 and mostly recovered within 72 hours, the PSII activity of dry cortex-removed samples decreased from 0.62 to 0.4 and recovered to 0.55 within 72 hours. The wet samples were reduced from 0.7 to 0.2 (intact) and from 0.62 to 0.25 (removed cortex). Within the 72 hours revitalization period, the intact thalli samples recovered slightly to 0.25, while no recovery was observed at cortex-removed samples. The maximum UV-C dose affected the dry samples as described before, the PSII activity was reduced from 0.68 to 0.55 (intact) and from 0.6 to 0.5 (removed cortex) and recovered within 72 hours to 0.65 and 0.58, respectively (Fig. 6C). In both wet sample sets, the PSII activity was dramatically reduced from 0.68 to 0.02 (intact) and from 0.6 to 0.08 (cortex removed). While the wet and intact samples recovered to 0.3 within 72 hours, no recovery was observed with the removed-cortex samples.

Fig. 6. Recovery capacity of the photosystem II (PSII) of the Trebouxia sp. photobiont of Rhizocarpon geographicum (Fig. 6A–C) and Circinaria gyrosa (Fig. 6D–F) after three selected UV-C irradiation doses and under the four different experimental conditions. Mean value±standard deviation given in each figure.
All R. geographicum samples seemed to remain unaffected after exposure by the lowest UV-C dose. These samples showed a steady pattern of PSII values after 24, 48 and 72 hours of revitalization, similar to pre-exposure data. After medium and highest UV-C doses, the data revealed succinct differences: intact-dry thalli displayed best photosynthetic performance and recovery. A comparable reaction was detected in dry samples with removed cortex. Wet samples displayed lower PSII values and a diminished resilience during revitalization presumably demonstrates severe damages induced by UV-C.
‘Circinaria gyrosa’
The thallus samples of C. gyrosa showed a less complex pattern of PSII activity during revitalization. Dry thallus samples (intact as well as acetone-rinsed) were virtually not affected by the three UV-C doses tested. The post-exposure values did not differ significantly from the respective pre-exposure data, all ranging between ∼0.6 and ∼0.7 QY (Fig. 6D–F). In the correspondent wet samples neither significant loss of PSII activity nor recovery was observed within the 72 hours revitalization period. The PSII activity of wet intact thalli was not affected by the lowest UV-C dose, but reduced from ∼0.65 to ∼0.5 by the two following doses (Fig. 6D–F). The PSII activity of wet acetone-rinsed thalli was slightly reduced from 0.67 to 0.6 by the lowest dose, from 0.62 to 0.3 by the medium dose, and from 0.62 to 0.42 by the highest dose.
Comparison of both lichen photobionts
In all dry samples, the PSII activity of intact thalli was slightly higher compared with the respective processed ones. Comparing the changes of PSII activity of the two Trebouxia sp. photobionts, several differences should be highlighted: (1) Dry samples of R. geographicum showed a reduction of PSII activity after 24 hours revitalization that was recovered almost to the pre-exposure control values within 72 hours. (2) In contrast, dry samples of C. gyrosa were not affected by any of the three UV-C doses and thus showed no recovery. (3) Wet intact samples of R. geographicum showed some recovery after irradiation with 2.4 and 7.2×107 J m−2, while no recovery occurred in samples with removed cortex during the three-day period of revitalization. (4) In wet samples of C. gyrosa, there was also no observable recovery in the revitalization period but the reduction of PSII activity was more pronounced in acetone-rinsed thalli than in intact ones. (5) Despite the lack or retardation of PSII recovery in C. gyrosa, its average reduction of PSII activity after exposure is less pronounced at the two higher UV-C doses compared with R. geographicum.
Pigments content after UV-C exposure
‘Rhizocarpon geographicum’
In general, chl b was the most abundant pigment in R. geographicum's photobiont, followed by chl a, lutein and β-carotene. Compared with the controls (about 60–80 μg g−1 TDW), chl a and chl b revealed no correlation of UV-C dose/experimental condition and concentration when subjected to the lower doses of 2.5×106, 7.5×106 and 1.2×107 J m−2 (Fig. 7A–C), irrespective of the hydration state or the presence/absence of protective structures. In contrast, concentration values of β-carotene were more constant and comparable to the control (about 10 μg g−1 TDW). Lutein maintained its concentration constant and close to 40 μg g−1 TDW, while only the intact-dry sample at 7.5×106 J m−2 showed an elevated concentration of 110 μg g−1 TDW (Fig. 7B). Besides, the four experimental approaches did not influence the concentration of the two carotenoids. After the higher UV-C doses of 2.4, 4.8 and 7.2×107 J m−2 (Fig. 7D–F), a general decrease in the assessed pigments content was detected. In samples subjected to 2.4×107 J m−2 the concentration of chl a was decreased in the intact-wet sample only, while the subsequent doses caused a decrease of chl a in all experimental approaches (Fig. 7D–F). At a dose of 2.4×107 J m−2 the chl b concentration gave no clear pattern, while the higher doses elicited a decrease in the cortex-removed samples (Fig. 7E and F). The effect of the higher UV-C doses on β-carotene and lutein was similar, both revealing a dose-dependent decrease except in intact wet samples, which were virtually unaffected. In removed-cortex samples (dry and wet), the pigment concentrations of β-carotene and lutein were reduced to nearly zero and to about 10 μg g−1 TDW, respectively. The ratios of chl a/chl b, β-carotene/chl a+b and lutein/chl a+b were calculated. Due to the high variability of chl a and chl b concentrations among all UV-C doses and experimental approaches, these ratios gave no indication of distinctive degradation processes by UV-C exposure. The β-carotene/chl a+b ratios were stable but always low, due to the low abundance of β-carotene in all samples.

Fig. 7. Content of chlorophyll a, chlorophyll b, β-carotene and lutein per gram of lyophilized biomass in R. geographicum and quantified by HPLC. Pigment content quantified by HPLC, n=2 replicates in intact and rinsed control (non-irradiated) samples represented as mean value±standard deviation.
‘Circinaria gyrosa’
In C. gyrosa's photobiont, the same relative abundance as in R. geographicum was observed for the four quantified pigments (chl b>chl a >lutein>β-carotene), but with less marked differences between chl b and chl a contents and very low levels of β-carotene (0–10 μg g−1 TDW). After exposure to the lowest UV-C dose, chl a as well as chl b concentrations revealed lower levels in dry and wet intact samples but elevated levels in dry and wet rinsed samples when compared with the control (chl a: 32 μg g−1, chl b: 42 μg g−1). After exposure to 7.5×106 J m−2 (Fig. 8B) the concentration of both chlorophylls was comparable to the control, except a in the intact-wet samples. With the next two UV-C doses (1.2 and 2.4×107 J m−2), chl a and chl b both displayed an increase in the intact-dry samples (1.5–3-fold) and a decrease of ∼50% in the rinsed-dry samples (Fig. 8C and D). In contrast, the chl a and chl b values obtained in the samples irradiated with 4.8×107 J m−2 were similar to the respective controls for intact samples (70 μg g−1 TDW) and rinsed samples (60 μg g−1 TDW, Fig. 8E). Finally, the samples submitted to 7.2×107 J m−2 showed a different pattern depending on the experimental approaches. Compared with their control, chl a and chl b concentrations in dry intact and dry rinsed samples remained constant while they decreased in both wet samples (Fig. 8F).

Fig. 8. Content of chlorophyll a, chlorophyll b, β-carotene and lutein per grams of lyophilized biomass in C. gyrosa and quantified by HPLC. Pigment content quantified by HPLC, n=2 replicates in intact and rinsed control (non-irradiated) samples represented as mean value±standard deviation.
The four experimental approaches, as rinsing and hydration state, had no effect on β-carotene and lutein. After exposure to the three lower UV-C doses, lutein and β-carotene concentrations were comparable to their controls (Fig. 8A–C). After being exposed to 2.4 and 4.8×107 J m−2, none of the pigments was virtually influenced and also the maximum UV-C dose of 7.2×107 J m−2 displayed no appreciable influence on β-carotene and lutein (Fig. 8D–F).
As in R. geographicum, the chl a/chl b ratio displayed high variability, while being more constant than in R. geographicum. Except one runaway value in the intact-dry sample (4.5 after 7.2×107 J m−2) all chl a/chl b ratios ranged from 0.5 to 0.7. The β-carotene/chl a+b ratio showed again the lowest (close to 0) but most constant values among all UV-C doses and experimental approaches. The lutein/chl a+b ratio in samples exposed to 2.5×106 and 7.5×106 J m−2 irradiations was ∼0.25 with no clear influence of the rinsing and the hydration state. At higher doses, the lutein/chl a+b ratio showed some variations without a clear trend depending on the applied dose or the experimental condition.
Comparison of both lichen photobionts
In both Trebouxia sp. photobiont chl b was found to be the most abundant pigment, followed by chl a, lutein and β-carotene. The overall content of chlorophylls was slightly higher in R. geographicum compared with C. gyrosa. The results obtained in C. gyrosa's photobiont were more constant than the ones obtained in R. geographicum. Concerning the calculated ratios between chl a/chl b, β-carotene/chl a+b and lutein/chl a+b for both species after the same UV-C irradiation doses and under equivalent experimental conditions, it was observed that the ratio differences between β-carotene/chl a+b and lutein/chl a+b were always similar and that chl a/chl b was the one showing highest variability.
Discussion
Organisms are able to use two decisive strategies to endure excessive levels of UVR and to reduce its harmful effects on metabolism: to repair UVR-induced damage (as discussed in the introduction) or to avoid that damage (Björn Reference Björn2007). As the first possibility has been covered in the introduction we will discuss now the second strategy. Damage avoidance is proposed as an alternative mechanism to explain high levels of UVR resistance. According to the results of the present study, we suggest that the poikilohydric character of lichens is the major avoidance mechanism to explain the high tolerance towards UV-C in R. geographicum and C. gyrosa. Poikilohydry is the capacity to tolerate extreme desiccation during extended periods, recovering under more favourable conditions without physiological damage. During this ‘anabiotic’ state lichens are better disposed to resist harsh environmental parameters such as extreme temperatures (Kranner et al. Reference Kranner, Beckett, Hochman and Nash2008) or high levels of PAR and UVR (Nybakken et al. Reference Nybakken, Solhaug, Bilger and Gauslaa2004). It occurs in organisms that cannot regulate their internal water content, depending exclusively on the environmental conditions (Green and Lange Reference Green and Lange1994). The current study demonstrates for both Trebouxia sp. photobionts that the PSII is much more sensitive towards UV-C when the lichen thallus is wet, starting to decrease on their PSII at a dose of 5.0×106 J m−2. From these results it could be concluded that the dissociation of the PSII and its light harvesting complexes (LHC) during desiccation (Lange et al. Reference Lange, Bilger, Rinke and Schreiber1989) and the associated photoprotection mechanisms given by thermal dissipation and/or charge separation in the reaction centres (Heber et al. Reference Heber, Soni and Strasser2011) might prevent the putative degradation of the D1/D2 complex. Such mechanism might serve as an explanation for the high UV-C resistance of the two tested photobionts when dry. UV-C damage on PSII activity occurs in wet thalli, what might be explained by the re-association of PSII and LHC and thus the degradation of the D1/D2 complex or by degradation of chlorophylls (Strid et al. Reference Strid, Chow and Anderson1994; Teramura & Sullivan Reference Teramura and Sullivan1994), both by excess shortwave light energy. The present results on PSII performance clearly demonstrates that both photobionts are more vulnerable when metabolically active. This is in line with a study on X. elegans and P. aphthosa (de Vera & Ott Reference de Vera, Ott, Seckbach and Grube2010b), where a decrease of Trebouxia-photobiont viability was registered after UV-C exposure. The maximum dose applied during the present study is almost 500 times higher than the highest one used in the previously referred work. Considering such high a tolerance, the UV-C resistance expectancy of Trebouxia sp. has been largely increased. In metabolically active samples of R. geographicum, the PSII tolerance limit towards UV-C was observed at a dose of 4.8×107 J m−2 when the cortex was previously removed. We suppose that this dose is the upper limit of photosynthetic resistance of Trebouxia sp. to UV-C radiation. These results clearly expand the previous view that the photobiont is the more sensitive partner of the lichen symbiosis. In the ecological study performed by Sadowsky & Ott (Reference Sadowsky and Ott2012), some photobiont-specific physiological adaptations were found in Antarctic lichenized Trebouxia sp. suggesting a genetic basis for high tolerance. In the case of C. gyrosa, we were not able to detect its tolerance limit.
Protective thallus structures and light-screening SLCs are generally considered as crucial adaptations towards extreme environmental parameters as UVR (Fahselt Reference Fahselt1994; Rikkinen Reference Rikkinen1995; Huneck Reference Huneck1999) and also explain their high resistance demonstrated in previous astrobiological studies (Sancho et al. Reference Sancho, de la Torre and Pintado2008; Meeßen et al. Reference Meeßen, Sánchez, Brandt, Balzer, de la Torre, Sancho, de Vera and Ott2013). In some field campaigns that compared the PSII performance of samples of R. geographicum with and without cortex (de la Torre Noetzel Reference de la Torre Noetzel2002), it was demonstrated that the cortex has an important protective function in physiologically active thalli. Accordingly, the lichen's cortex – which gives mechanical protection as well as excess PAR and/or UVR-screening effects by the cortex-located SLC rhizocarpic acid – was proposed to be the main reason to explain the high resistance of R. geographicum to the full spectrum of UVR (de la Torre et al. Reference de la Torre Noetzel, Sancho, Pintado, Rettberg, Rabbow, Panitz, Deutschmann, Reina and Horneck2007a, Reference de la Torre2010). Nonetheless, during this study, the removal of the cortex of R. geographicum did not lead to a significant loss of PSII activity in the UV-C exposed algal layer when dry, indicating that the anhydrobiotic condition has the main protective function. The acetone-rinsing of C. gyrosa – to extract putative photoprotectant SLCs – did not evoke any differences in PSII activity. Concerning the revitalization measurements, dry exposed thalli as well as wet intact thalli of R. geographicum show some recovery of the UV-C affected PSII during the revitalization period. Wet thalli with removed cortex did not show any recovery. Concluding from these results the protective effect of the cortex and cortex-located SLCs may be more important in periods when metabolic activity and UV-C irradiation co-occur.
Despite the higher resistance of the PSII performance of metabolically active photobionts in C. gyrosa (Fig. 5B), no recovery of the PSII activity occurred in the revitalization period (Fig. 6E and F). Raggio et al. (Reference Raggio, Pintado, Ascaso, de la Torre, de los Ríos, Wierzchos, Horneck and Sancho2011) confirmed previous results as summarized in Culberson (Reference Culberson1979) and after looking for SLCs by thin layer chromatography assays in samples of C. gyrosa, no SLC at all was found. Meeßen et al. (2013) suggested that the dense and highly gelatinated subcortex between the SLC-deficient cortex and the algal layer may play an important role in the high resistance of C. gyrosa towards UV-C and act as a secondary adaptation to block radiation and compensate the lack of SLCs. This interpretation might be fostered by the present finding that the PSII activity was not affected in acetone-rinsed thalli, where putative SLCs have been removed. As the subcortex (Fig. 1, B2) was not affected by the acetone-rinsing procedure, the protective effect of the subcortex can be assumed to be given in both experimental set-ups.
Comparing the results of the single factor UV-C irradiation in the present study with previous multi-factor space exposure experiments is not easy: intact and dry R. geographicum showed no loss of PSII activity in pre- and post-exposure measurements (Fig. 4A) after 67d space-equivalent UV-C and a slight loss in the revitalization experiment (Fig. 6C). After 1.5 years of space exposure in the LIFE experiment (Onofri et al. Reference Onofri, de la Torre, de Vera, Ott, Zucconi, Selbmann, Scalzi, Venkateswaran, Rabbow, Sánchez Iñigo and Horneck2012) there was a dramatic loss of PSII activity down to 0.5% of the pre-flight value. Thus, the loss of PSII activity in LIFE can be rather explained by the 8-times longer exposure and accumulation of PSII damages or by the more deleterious effect of multi-factor space exposure than by mere UV-C exposure. If the cortex of R. geographicum is removed, the slight loss of PSII activity of about 8% after 67d space-equivalent UV-C (Fig. 4C) is comparable to the loss of PSII activity of about 4–10% after 10 days in the space experiment Lithopanspermia (dry thalli with removed cortex, de la Torre et al. Reference de la Torre2010), indicating that a cumulative damaging effect on PSII after 10 days in LEO is roughly comparable to a single factor UV-C dose of 67 days space-equivalent. In dry samples of C. gyrosa, no loss of PSII activity was observed after 67 days space-equivalent UV-C doses (Figs. 5A and 6D–F). With an intermediate UV-C dose, the results are consistent with the minimal loss of PSII activity after 10 days in space (from 100% to 95–99%, de la Torre et al. Reference de la Torre2010) and the loss of about 55% of PSII activity after 1.5 years in the LIFE experiment (Onofri et al. Reference Onofri, de la Torre, de Vera, Ott, Zucconi, Selbmann, Scalzi, Venkateswaran, Rabbow, Sánchez Iñigo and Horneck2012), indicating again that pure UV-C is not as damaging as multi-factor space exposure.
In relation with the pigments analyses performed, it should be taken into account that while chl a and chl b are basic photosynthetic molecules that allow plants to absorb energy from light, carotenoids are a group of organic pigments present in photosynthetic organisms and involved in two main functions: accessory absorption of light energy for photosynthesis and protection of chlorophylls from damage by quenching excess light energy of non-PAR sunlight (Paerl Reference Paerl1984; Armstrong & Hearst Reference Armstrong and Hearst1996). A potential role of carotenoids in UV protection has been proposed, especially having in mind that β-carotene can absorb UV-C (Holder Reference Holder1998) although further work is needed until that protective role can be confirmed (Wynn-Williams & Edwards Reference Wynn-Williams, Edwards, Horneck and Baumstark-Khan2002b). Concerning the post UV-C-irradiation quantification of pigments performed, we should differentiate between two different perspectives: the relative abundance of photosynthetic pigments and the effect of UV-C on them.
Normally the content of chl a in the chloroplast is higher compared with chl b (Atwell et al. Reference Atwell, Kriedemann and Turnbull1999). Both chlorophylls are involved in light-harvesting but with important differences. While chl a is directly implicated in the energetic processing at high-light levels (higher chl a/chl b ratios), chl b plays a major role in the optimization of the photosynthetic process under low light conditions enhancing the efficiency of blue light absorption (Atwell et al. Reference Atwell, Kriedemann and Turnbull1999; Yamazaki et al. Reference Yamazaki, Takahisa, Emiko and Yasumaro2005). Accordingly, the generally lower chl a/chl b ratios obtained during this study indicate that both Trebouxia sp. photobionts are adapted to low light. This conclusion contradicts the light conditions of their natural habitat, as R. geographicum and C. gyrosa are species subjected to high levels of insolation. However, we assume that both lichen species have developed efficient structures to drastically reduce the amount of light reaching the photosystems, as the combination of their usual anhydrobiotic state (to avoid physiologically stressful conditions), and effective shielding structures as cortices, subcortices and SLCs. No direct correlation between key-photosynthetic pigments chl a and chl b concentration and a higher PSII activity was detected for any lichen species.
In relation with the effect of UV-C on the assessed pigments, no clear trend in the levels of chl a and chl b or of the carotenoids β-carotene and lutein has been observed after the different UV-C irradiation doses and experimental conditions. Nonetheless, the appearance of non-identified peaks next to chlorophyll and carotenoids signals in the HPLC chromatograms of the samples subjected to the highest UV-C doses might point to a degradation of chlorophyll and carotenoids by the oxidative action of UV-C, as proposed by Gao et al. (Reference Gao, Cui, Xiong, Li and Wu2009). In that respect, chlorophylls and carotenoids might also be contributing to preserve the photobiont from the damaging effects of UV-C. This protective activity would be of physico-chemical nature and would consist of the energy absorption through the high electron densities located in the double bounds. Whether or not the carotenoids pigments play a special role in the UV-C tolerance detected in both Trebouxia sp. photobionts is unclear, but previous research on lichen photobionts reported an increase in the carotenoid content after exposure to UV-B as a protective mechanism (Buffoni Hall Reference Buffoni Hall2002; Gautam et al. Reference Gautam, Singh and Pant2011). Similar results were obtained with some microalgae species after exposures to UV-A (Salguero et al. Reference Salguero, León, Mariotti, de la Morena, Vega and Vílchez2005; Mogedas et al. Reference Mogedas, Casal, Forján and Vílchez2009) and explained as an antioxidant-protective mechanism towards UV-B and UV-A. In contrast, a decrease in the content of some of the quantified pigments has been detected after the highest UV-C doses, especially in the samples of R. geographicum with removed cortex after doses of 4.8 and 7.2×107 J m−2 (Fig. 7E and F respectively). The general decrease in the chlorophyll, β-carotene and lutein content in those samples could be caused by UV-induced degradation, as explained above, fostered by the absence of the protective cortex. On the contrary, after the same irradiations upon intact samples no degradation took place but a slight increase in the wet and active exemplars was detected, reinforcing the idea of the decisive role of cortical structure and physiological state in the UV-C tolerance of R. geographicum. In some cases, an increase of photosynthetic pigment concentration was detected in dry (anabiotic) samples of both lichen species after different UV-C exposures. These results could be either explained by UV-C induced pigment synthesis during the 72 hour-revitalization or by high intersample variability. The lichen species C. gyrosa maintained a more stable pigment content after all UV-C irradiations and experimental conditions, highlighting again the protective role of the subcortex.
In summary, the tolerance expectancy of Trebouxia sp. within the lichen species R. geographicum and C. gyrosa towards UV-C, one of the most critical space factors, has increased considerably compared with previous results. Both photobionts have demonstrated high tolerance in terms of PSII activity, clearly influenced by the physiological state of the thallus. As previously hypothesized, photobiont PSII activity is more resistant to UV-C radiation in the anabiotic state, showing decreasing levels of activity when wet and physiologically active. Besides the key anhydrobiosis-mediated resistance that underlies the UV-C tolerance detected for both studied species, the presence of passive protective structures is proposed here as a complementary mechanism to explain the high UV-C tolerance detected. In R. geographicum that protective structure is the thick epicortex with densely arranged cortical cells that are intensely coloured and incrusted with SLCs (Fig. 1, A2 and A3) and in C. gyrosa it is the dense and highly gelatinated subcortex (Fig. 1, B2 and B3 refer to Meeßen et al. Reference Meeßen, Sánchez, Brandt, Balzer, de la Torre, Sancho, de Vera and Ott2013). These structures have an important role in blocking excess radiation and act as a primary protective adaptation in highly insolated habitats. The poikilohydric character of lichens and the presence of protective structures are the main reasons to explain their tolerance in relation with light stress that have leaded them to tolerate even highly energetic wavelengths that do not reach the Earth's surface. As UV-C does not occur under terrestrial conditions, resistance towards UV-C should be considered as a secondary effect.
Further research should be done with isolated photobionts in order to evaluate their tolerance capabilities, and we hypothesize that they will result in lower rates of tolerance and survivability after the exposure to similar conditions. In conclusion, we support the idea proposed by Sancho et al. (Reference Sancho, de la Torre and Pintado2008) that among extremotolerant organisms, lichens possess a diverse set of qualities that make them excellent objects for astrobiological research.
Acknowledgements
The first author would like to express his sincere gratefulness to Mr Gilberto Herrero (Geology Faculty, Stratigraphy Department, Universidad Complutense de Madrid – UCM) for his generous technical support during the Rhizocarpon geographicum samples edition and to Mrs Eva-Maria Balzer (Institute of Botany, Heinrich Heine Universität, Düsseldorf) for the realization of ultra-thin sections for the light and SEM microscopes with her excellent skills. S. Ott and J. Meeßen express their gratitude to the German Federal Ministry of Economics and Technology (BMWi) and the German Aerospace Center (DLR) for funding (50BW1153). We also thank the anonymous reviewers for their valuable comments and suggestions for improvement. The first author was recipient of a PhD scholarship during the development of this article given by the Instituto Nacional de Técnica Aeroespacial – INTA and this research work will be included in his PhD thesis.














