Overweight and obesity are a real health problem in the twenty-first century. According to the International Obesity Task Force, there are more than 1000 million overweight adults in the world, of whom at least 475 million are obese(1). Although not exclusively, the phenomenon of obesity in present-day society is largely due to changes in lifestyle: (1) high-fat feeding; (2) high snacking frequency; (3) reduction in the total daily sleep period; (4) increase in the exposure to bright light during the night; (5) nocturnal feeding(Reference Delezie and Challet2). This background induces the brain to lose its perception of internal and external rhythms, and therefore to disrupt its circadian rhythms.
In mammals, the circadian timing system is composed of several endogenous clocks. The main component of this circadian system is the suprachiasmatic nucleus of the hypothalamus, but circadian clocks are also present in most peripheral tissues as is the case of adipose tissue(Reference Kohsaka and Bass3, Reference Reppert and Weaver4). Recent research has revealed relationships between adipose tissue clock gene desynchronisation and the development of certain diseases, such as obesity(Reference Turek, Joshu and Kohsaka5–Reference Doi7).
Input signals, such as light (light/dark changes) or meal times (intake/fast), set the clock of the mammal circadian system. Hence, the modification of these signals leads to clock gene disruption. It has been well reported that different periodicities in constant exposure of mice or human subjects to bright or dim light lead to a disruption between internal and external rhythms and are connected with a larger body mass gain and increased insulin:glucose ratio(Reference Karatsoreos, Bhagat and Bloss8, Reference Martino, Tata and Belsham9). Similarly, elevated nocturnal feeding has been correlated with an increased risk of overweight in some studies(Reference Colles, Dixon and O'Brien10, Reference Lundgren, Smith and Spresser11). Different publications have also confirmed the metabolic consequences (gain of more body mass) of unusual feeding timing(Reference Mistlberger, Lukman and Nadeau12, Reference Kohsaka, Laposky and Ramsey13).
As mentioned previously, high-fat feeding, one of the characteristic unhealthy lifestyle choices in developed countries, is partially responsible for increased obesity. Several articles have pointed out that it produces progressive derangements in temporal communication among different food intake signals by modifying the strength, duration and frequency of circadian rhythms(Reference Cha, Chou and Boozer14, Reference Havel, Townsend and Chaump15). In this context, it has been demonstrated that high-fat feeding causes a phase delay in adiponectin circadian rhythm. Conversely, fasting has opposite effects, resulting in a phase advance(Reference Barnea, Madar and Froy16, Reference Barnea, Madar and Froy17). Thus, it could be proposed that fasting or energy restriction could reverse circadian rhythmicity disruption in clock genes caused by high-fat feeding.
Scientific research is constantly working to find new molecules, either drugs or ingredients in the diet, which are effective in preventing excess accumulation of body fat and associated complications. This is the case of trans-resveratrol (3,4′,5-trihydroxystilbene), a polyphenol present in different foods (berries and some nuts) and beverages of plant origin (wine and juice)(Reference Frémont18). The effects of this molecule on circadian rhythmicity in adipose tissue have not been previously studied; however, considering that many health benefits associated with resveratrol have been attributed to its ability to mimic the effects of a energy-restrictive diet(Reference Barger, Kayo and Vann19), resveratrol may be a useful tool in avoiding circadian disruption caused by high-fat feeding. The evidence that resveratrol regulates the expression of clock genes period homologue 1 (Per1), Per2 and aryl hydrocarbon receptor nuclear translocator-like (Bmal1) in Rat-1 fibroblast cells(Reference Oike and Kobori20) is in good accordance with this proposal.
Thus, the purpose of the present study was to analyse the potential effect of resveratrol on changes induced by high-fat feeding in the expression of different clock genes and clock-controlled genes in white adipose tissue.
Materials and methods
Animals, diets and experimental design
Male Wistar rats (Harlan Ibérica), 6 weeks old, were individually housed in polycarbonate metabolism cages (Tecniplast Gazzada). The animals were housed in a temperature-controlled facility (22 ± 2°C) and maintained under a light–dark cycle with 12 h light and 12 h darkness per d (lights on 22.00 hours, lights off 10.00 hours). The experiment took place in accordance with the institution's guide for the care and use of laboratory animals (CUEID CEBA/30/2010). After a 6 d adaptation period, the animals were randomly divided into three groups (n 8) and fed experimental diets for 6 weeks. The experimental diets were supplied by Harlan Ibérica. The control group was fed a commercial standard diet. Of these experimental groups, one (control group) was fed a commercial standard diet (reference TD.06 416), which provided 15·5 kJ/g (3·7 kcal/g) and 10 % of energy as fat. The other two groups, high-fat group (HF) and resveratrol group (RSV), were fed a commercial high-fat diet (reference TD.06 415), which provided 19·3 kJ/g (4·6 kcal/g) and 45 % of energy as fat. In the RSV group, resveratrol was daily added to the fresh diet, as described previously(Reference Macarulla, Alberdi and Gómez21), in amounts that assured a dose of 30 mg/kg body weight per d. The diet was provided to rats at 10.00 hours, when the dark phase started in the animal facility room. All animals had free access to the diet and water.
Body weight and food intake were measured daily. At the end of the experimental period, rats were killed under anaesthesia (chloral hydrate) by cardiac exsanguination at the beginning of their activity phase, between 10.00 and 12.00 hours (from 0 to 2 h after dark clock onset). Although it is well known that rats eat mainly during the dark phase, in order to avoid any potential differences among them induced by spontaneous feeding, and to mimic the natural feeding rhythm of animals, all rats were fasted during the last 12 h before killing. Adipose tissues from the epididymal, perirenal, mesenteric and subcutaneous regions were dissected and weighed and then immediately frozen. All samples were stored at − 80°C until analysis.
The experimental procedure used in the present study followed the guidelines of the Animal Usage of the University of Basque Country (CUEID CEBA/30/2010).
RNA extraction and quantitative real-time PCR
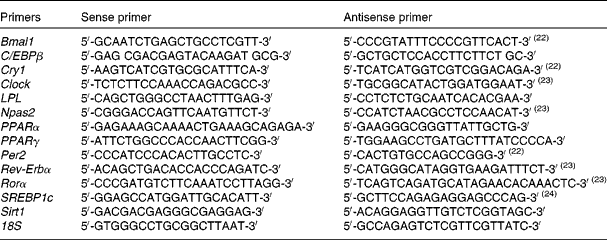
Total RNA was isolated from the epididymal adipose tissue and liver using Trizol (Invitrogen) according to the manufacturer's instructions. After DNase treatment (Ambion; The RNA Company, Applied Biosystems), 1·5 μg of total RNA were reverse transcribed into complementary DNA (iScript cDNA Synthesis Kit; Bio-Rad) according to the manufacturer's instructions. A 9·5 μl aliquot of each diluted complementary DNA sample was used for PCR amplification in a 25 μl reaction volume. The complementary DNA samples were amplified on an iCycler-MyiQ real-time PCR detection system (Bio-Rad) in the presence of SYBR Green master mix (Applied Biosystems) and a 300 nm concentration of each of the sense and antisense primers. Specific primers were synthesised commercially (Integrated DNA Technologies), and the sequences are listed in Table 1(Reference Farnell, Allen and Nahm22–Reference Orolin, Vecera and Markova24). mRNA levels in all samples were normalised to the values of 18S and the results expressed as fold changes of the threshold cycle (C t) value relative to the controls using the ![]() $$2^{ - \Delta \Delta C _{t}} $$ method(Reference Livak and Schmittgen25).
$$2^{ - \Delta \Delta C _{t}} $$ method(Reference Livak and Schmittgen25).
Table 1 Primers for PCR amplification of each gene studied (SYBR® Green RT-PCR)

Bmal, aryl hydrocarbon receptor nuclear translocator-like; C/EBP, CCAAT/enhancer-binding protein; Cry, cryptochrome; Clock, clock homologue; LPL, lipoprotein lipase; Npas, neuronal PAS domain protein; Per, period homologue; Rev-Erb, reverse erythroblastosis virus; Ror, retinoid-related orphan receptor; SREBP, sterol regulatory element-binding protein; Sirt, sirtuin.
Western blot
Epididymal nuclear proteins were extracted and quantified according to the manufacturer's instructions (Nuclear Extraction Kit; Cayman Chemical Company). Immunoblot analysis was performed using 20 μg of nuclear extracts separated by electrophoresis in a 10 % SDS–polyacrylamide gel and transferred to polyvinylidene difluoride membranes. Phospho-reverse erythroblastosis virus (Rev-Erbα Ser55/59) levels were detected via specific antibody (1:1000; Cell Signaling Technology).
Fatty acid synthase activity
For the analysis of fatty acid synthase activity, 1 g of epididymal adipose tissue was homogenised in 5 ml of buffer (pH 7·6) containing 150 mm-KCl, 1 mm-MgCl2, 10 mm-N-acetyl-cysteine and 0·5 mm-dithiothreitol. After centrifugation at 100 000 g for 40 min at 4°C, the supernatant fraction was used for the analysis. Enzyme activity was measured by using a spectrophotometric method, as described previously(Reference Miranda, Churruca and Fernández-Quintela26), and expressed as nmol NADPH consumed/min per mg protein.
Statistical analysis
Results are presented as means with their standard errors. Statistical analysis was performed with SPSS Statistics 19.0 (IBM, Inc.) using one-way ANOVA, followed by Tukey's post hoc test. For body weight and energy intake time courses, ANOVA with repeated measures were used. Significance was assessed at the P< 0·05 level.
Results
Body weight and adipose tissue weights
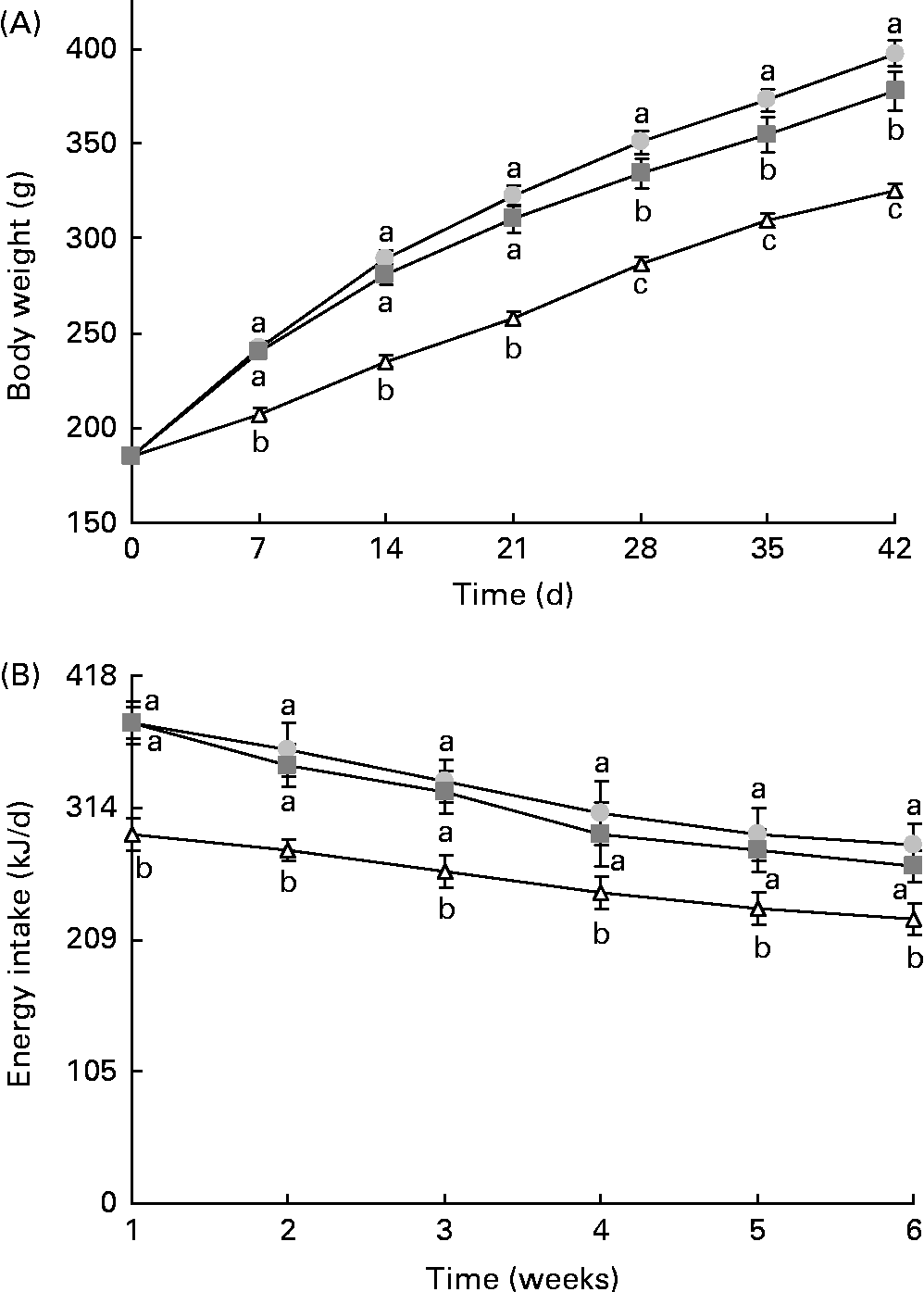
The final body weight and adipose tissue weights were significantly higher in animals from the HF groups than those from the control group (Fig. 1(A) and Table 2). Resveratrol significantly reduced body weight and the sum of adipose tissues, but these parameters did not reach the values observed for the control rats (Fig. 1(A) and Table 2). Although no significant differences in terms of food intake (g) were found between the control rats and rats fed the high-fat diets (control v. HF and RSV), energy intake was significantly higher in these rats than in the control rats. However, no differences in this parameter were found between the rats from the HF and RSV groups (Fig. 1(B)).

Fig. 1 (A) Body weight and (B) energy intake time courses in rats fed on the experimental diets for 6 weeks. Values are means for eight rats per group, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05). ![]() , Group fed a commercial standard diet, used as a control;
, Group fed a commercial standard diet, used as a control; ![]() , group fed a commercial high-fat diet;
, group fed a commercial high-fat diet; ![]() , group fed a commercial high-fat diet in which resveratrol was added daily in amounts that ensured a dose of 30 mg/kg body weight per d.
, group fed a commercial high-fat diet in which resveratrol was added daily in amounts that ensured a dose of 30 mg/kg body weight per d.
Table 2 Liver weight and adipose tissue weights in rats fed on the experimental diets for 6 weeks (Mean values with their standard errors, n 8 animals per group)

HF, rats fed a commercial high-fat diet; RSV, rats fed a commercial high-fat diet supplemented with resveratrol added daily in amounts that ensured a dose of 30 mg/kg body weight/d.
a,b,cMean values within a row with unlike superscript letters were significantly different.
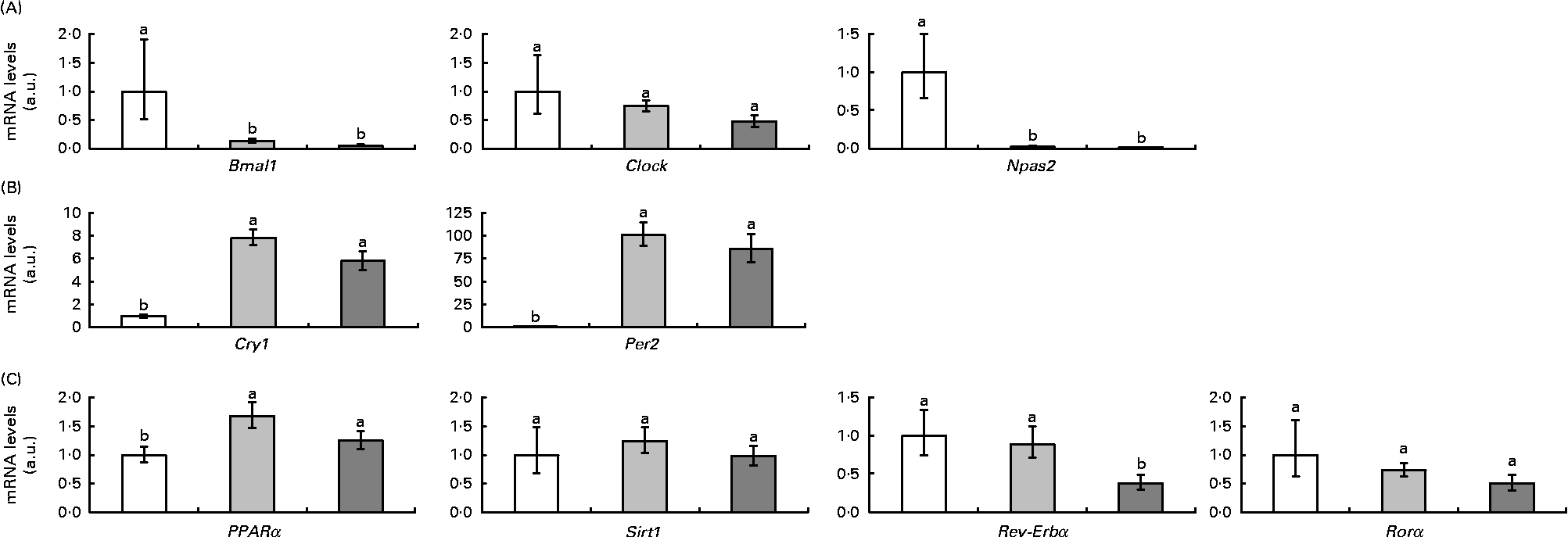
Clock genes and clock-controlled genes in adipose tissue
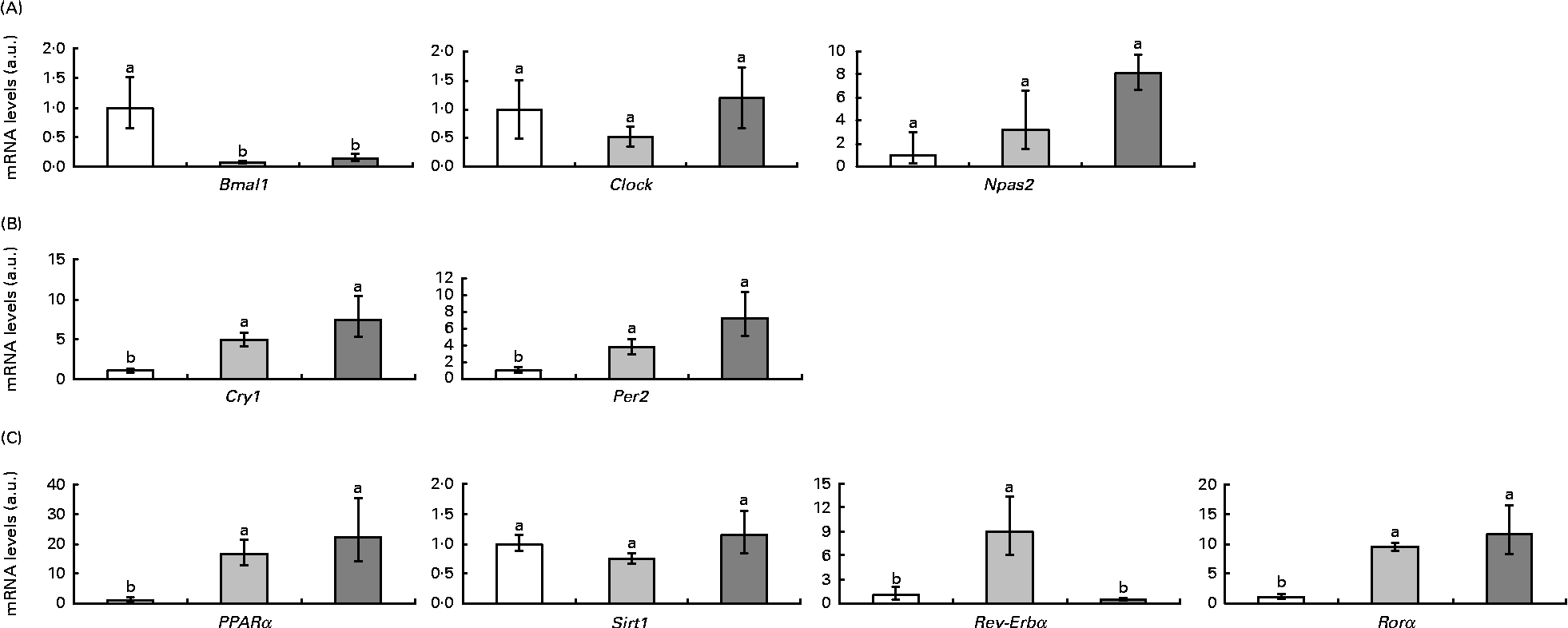
Fig. 2 represents clock and clock-controlled gene mRNA levels, expressed as values relative to the controls (control group), in the epididymal adipose tissue from rats in the HF and RSV groups. High-fat feeding led to the up-regulation of the negative elements (Per2 and cryptochrome 1 (Cry1)) and clock-controlled genes (PPARα, Rev-Erbα and retinoid-related orphan receptor (Rorα)), with the exception of sirtuin (Sirt1). With regard to the positive elements, Bmal1 was down-regulated and clock homologue (Clock) and neuronal PAS domain protein 2 (Npas2) remained unchanged. The RSV group showed a similar pattern of response to the HF group when compared with the controls, except for Rev-Erbα, which was down-regulated (P< 0·05). The expression of this gene reached the same levels as in the control rats.

Fig. 2 (A) Positive elements (aryl hydrocarbon receptor nuclear translocator-like (Bmal1), clock homologue (Clock) and neuronal PAS domain protein 2 (Npas2)), (B) negative elements (cryptochrome 1 (Cry1) and period homologue 2 (Per2)) and (C) clock-controlled genes (PPARα, sirtuin (Sirt1), reverse erythroblastosis virus α (Rev-Erbα) and retinoid-related orphan receptor α (Rorα)) mRNA levels expressed as values relative to the controls (control group), in the epididymal adipose tissue of rats fed on the experimental diets for 6 weeks (□, group fed a commercial standard diet, used as a control; ![]() , group fed a commercial high-fat diet;
, group fed a commercial high-fat diet; ![]() , group fed a commercial high-fat diet in which resveratrol was daily added in amounts that ensured a dose of 30 mg/kg body weight per d). Values are means for eight rats per group, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). a.u., Arbitrary units.
, group fed a commercial high-fat diet in which resveratrol was daily added in amounts that ensured a dose of 30 mg/kg body weight per d). Values are means for eight rats per group, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). a.u., Arbitrary units.
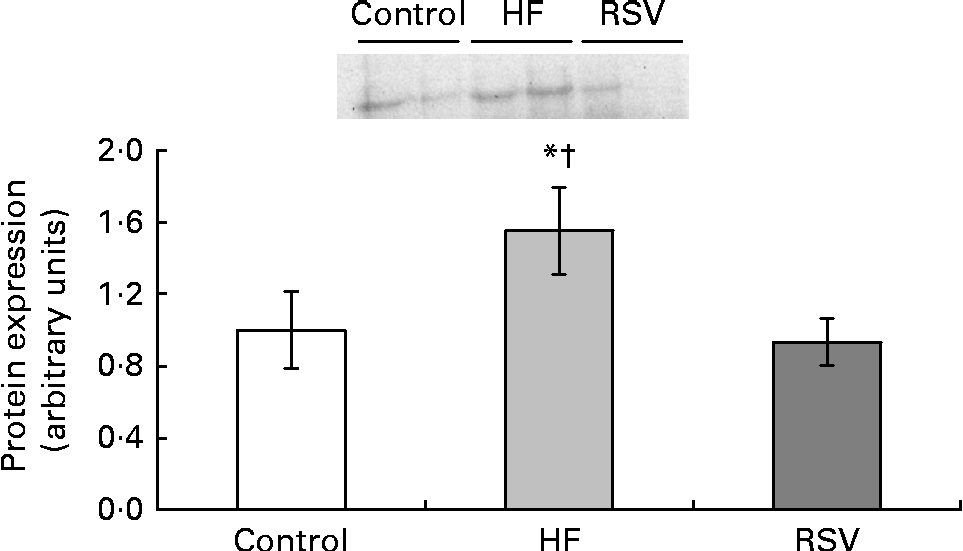
Due to the fact that Rev-Erbα was the only gene significantly modified by resveratrol, its protein expression was also analysed. The pattern of response of protein expression was similar to that found for gene expression, although statistical significance was not reached. Rats from the HF group showed increased values when compared with the control rats (50 %). By contrast, no differences were found between the control group and the RSV group (Fig. 3).

Fig. 3 Reverse erythroblastosis virus α levels of protein expression, presented as values relative to the controls (control group), in the epididymal adipose tissue of rats fed on the experimental diets for 6 weeks (control, group fed a commercial standard diet, used as a control; HF, group fed a commercial high-fat diet; RSV, group fed a commercial high-fat diet in which resveratrol was added daily in amounts that ensured a dose of 30 mg/kg body weight per d). Values are means for eight rats per group, with their standard errors represented by vertical bars. * Mean value was different from that of the control group (P< 0·2). † Mean value was different from that of the RSV group (P< 0·1).
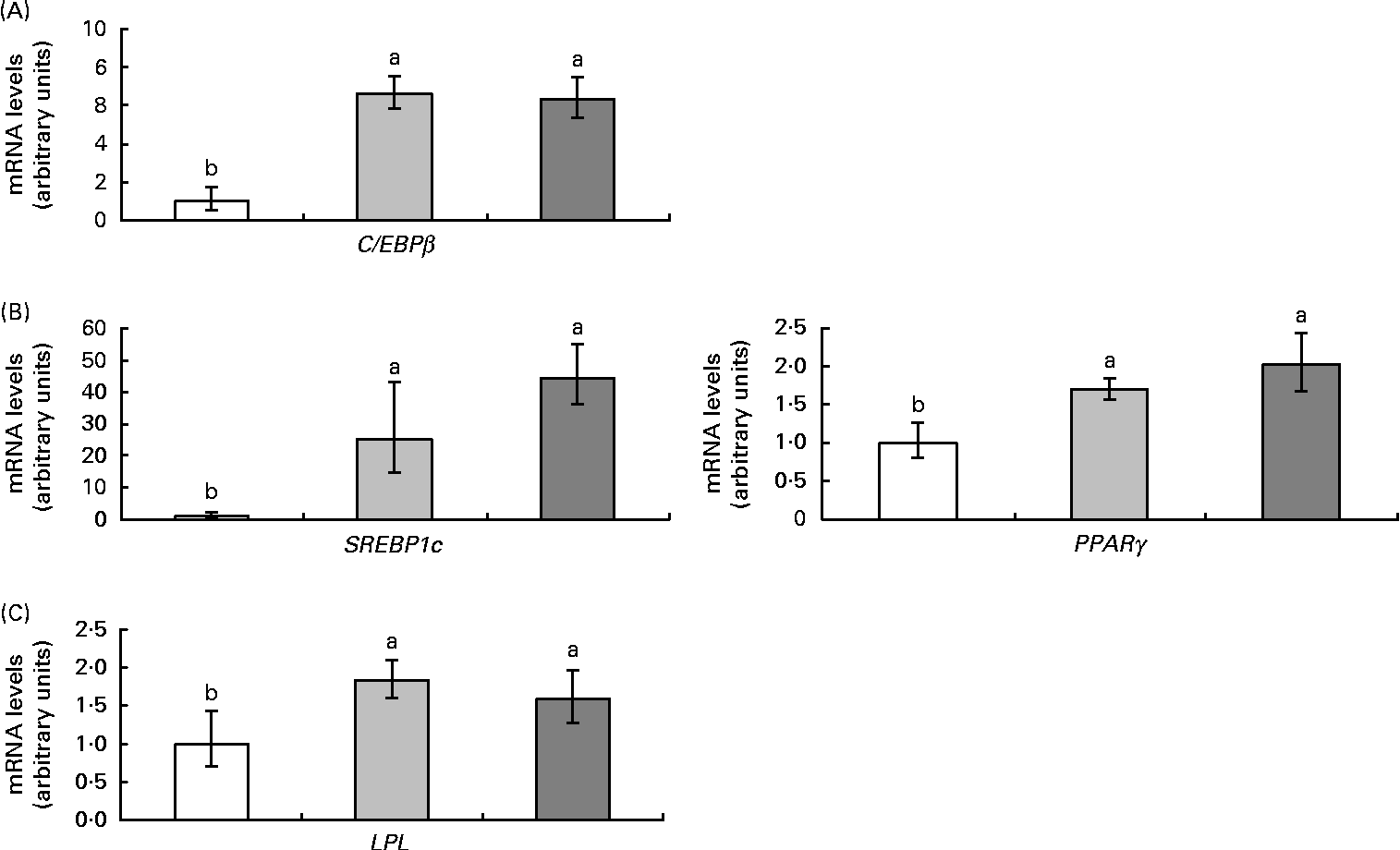
Parameters controlled by reverse erythroblastosis virus α in adipose tissue
Rev-Erbα is an important transcription factor in the control of adipogenesis and de novo lipogenesis. For this reason, the expression of the genes CCAAT-enhancer-binding protein (C/EBPβ), sterol regulatory element-binding protein (SREBP1c), PPARγ and lipoprotein lipase (LPL), as well as the activity of fatty acid synthase, were also analysed. High-fat feeding up-regulated all adipogenic genes and resveratrol did not affect this response (Fig. 4). As far as fatty acid synthase was concerned, high-fat feeding tended to increase its activity (P< 0·09) and resveratrol decreased it (P< 0·03) (Fig. 5).

Fig. 4 mRNA levels of genes ((A) CCAAT-enhancer-binding protein (C/EBPβ), (B) sterol regulatory element-binding protein 1c (SREBP1c) and PPARγ and (C) lipoprotein lipase (LPL)) related to adipogenesis in the epididymal adipose tissue from rats fed on the experimental diets for 6 weeks (□, group fed a commercial standard diet, used as a control; ![]() , group fed a commercial high-fat diet;
, group fed a commercial high-fat diet; ![]() , group fed a commercial high-fat diet in which resveratrol was added daily in amounts that ensured a dose of 30 mg/kg body weight per d). Values are means for eight rats per group, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05).
, group fed a commercial high-fat diet in which resveratrol was added daily in amounts that ensured a dose of 30 mg/kg body weight per d). Values are means for eight rats per group, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05).

Fig. 5 Fatty acid synthase (FAS) activity in the epididymal adipose tissue from rats fed a commercial high-fat diet in which resveratrol was added daily in amounts that ensured a dose of 30 mg/kg body weight per d. Control, group fed a commercial standard diet, used as a control; HF, group fed a commercial high-fat diet; RSV, group fed a commercial high-fat diet in which resveratrol was added daily in amounts that ensured a dose of 30 mg/kg body weight per d. Values are means for eight rats per group, with their standard errors represented by vertical bars. * Mean value was different from that of the control group (P< 0·09). † Mean value was significantly different from that of the RSV group (P< 0·03).
Clock genes and clock-controlled genes in the liver
In the liver, high-fat feeding down-regulated two positive elements, Bmal1 and Npas2, and up-regulated the negative elements and PPARα. Clock, Reb-Erbα, Rorα and Sirt1 gene expression remained unchanged. As in adipose tissue, resveratrol down-regulated (P< 0·05) the expression of Rev-Erbα in rats fed the high-fat diet (Fig. 6).

Fig. 6 mRNA levels of the analysed clock genes, (A) positive elements (aryl hydrocarbon receptor nuclear translocator-like (Bmal1), clock homologue (Clock) and neuronal PAS domain protein 2 (Npas2)) and (B) negative elements (cryptochrome 1 (Cry1) and period homologue 2 (Per2)), and (C) the clock-controlled genes (PPARα, sirtuin (Sirt1), reverse erythroblastosis virus α (Rev-Erbα) and retinoid-related orphan receptor (Rorα)) in the liver from rats fed on the experimental diets for 6 weeks (□, group fed a commercial standard diet, used as a control; ![]() , group fed a commercial high-fat diet;
, group fed a commercial high-fat diet; ![]() , group fed a commercial high-fat diet in which resveratrol was added daily in amounts that ensured a dose of 30 mg/kg body weight per d). Values are means for eight rats per group, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). a.u., arbitrary units.
, group fed a commercial high-fat diet in which resveratrol was added daily in amounts that ensured a dose of 30 mg/kg body weight per d). Values are means for eight rats per group, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). a.u., arbitrary units.
Discussion
The regulation of the peripheral clock and the clock-controlled genes present in the adipose tissue is still unclear(Reference Sukumaran, Xue and Jusko27). In the present study, we have analysed the effects of resveratrol on changes induced by high-fat feeding in a wide range of genes included in the clock machinery of adipose tissue: the positive elements of the clock (Bmal, Clock and Npas2), the negatives ones (Cry1 and Per2) and most of the clock-controlled genes, which connect the circadian clock with adipose tissue metabolism, such as Rorα, Rev-Erbα, Sirt1 and PPARα.
The effect of a high-fat diet on the expression of clock genes has previously been reported(Reference Kohsaka, Laposky and Ramsey13, Reference Barnea, Madar and Froy16, Reference Barnea, Madar and Froy17). Although the results obtained with regard to the expression of clock genes and clock-controlled genes in rats fed a high-fat diet were not part of the original scope of the present study, they do provide additional information as studies published up to date have been carried out in mice, while the present study was performed in rats. Moreover, these results are necessary to assess the effects of resveratrol on changes induced by high-fat feeding. It is difficult to make comparisons between previous studies and the present study due to the fact that, for example, in the study by Barnea et al. (Reference Barnea, Madar and Froy16, Reference Barnea, Madar and Froy17), mice were killed after a 24 h fast. Nevertheless, and in spite of this difference, in the present study, Bmal1 was altered by the high-fat diet, as Barnea et al. (Reference Barnea, Madar and Froy17) similarly observed in their studies. In addition, we also observed changes in the negative elements, as well as in most of the clock-controlled genes.
Despite the scientific community's attention to biomolecules in recent years because of their beneficial effects on health, very little is known about its potential effects on the clock system. Thus, having focused the present study on resveratrol, we have demonstrated for the first time that this biomolecule can reverse the effect of high-fat feeding on Rev-Erbα gene expression in the adipose tissue. We have also analysed the protein expression of this gene, finding a similar response pattern.
It is important not to forget that, as gene expression was measured only at one point, it is not possible to infer whether the observed differences among the experimental groups are due to a phase shift or to differences in gene expression degree. Nevertheless, this is a first approach to analyse whether clock machinery mediates the body fat-lowering effect of resveratrol, and therefore further studies should be carried out.
Rev-Erbα is a clock-controlled gene induced during adipogenesis(Reference Preitner, Damiola and Lopez-Molina28, Reference Fontaine, Dubois and Duguay29). In order to analyse whether changes observed in the present study in the expression of this gene were related to changes in adipogenesis, the expression of several genes involved in this metabolic process was examined. As expected, and in agreement with the literature(30), adipogenic genes were up-regulated in rats from the HF group, suggesting that, under high-fat feeding conditions, the up-regulation of Rev-Erbα was related to increased adipogenesis. By contrast, in comparison with the HF group, the down-regulation of Rev-Erbα observed in the RSV group was not accompanied by reduced adipogenesis as adipogenic genes remained unchanged.
Moreover, in the case of SREBP, Rev-Erbα participates not only in its gene expression regulation but also in its translocation to the nucleus(Reference Le Martelot, Claudel and Gatfield31), meaning that it regulates the maturation, and thus the activation, of this family of transcription factors. SREBP1c, which regulates adipogenesis, also plays a crucial role in the regulation of de novo lipogenesis by controlling the transcription of fatty acid synthase, a key enzyme in this metabolic process(Reference Amemiya-Kudo, Shimano and Hasty32). We analysed the activity of this enzyme in order to check whether in the present study the down-regulation induced by resveratrol in Rev-Erbα was related to reduced lipogenesis. We further observed that it followed the same pattern of response as Rev-Erbα in terms of both gene and protein expression. This means that enzyme activity was increased by high-fat feeding and decreased by resveratrol. According to these data, it can be proposed that resveratrol prevents fat accumulation induced by high-fat feeding by reducing lipogenesis in the adipose tissue, at least in part via Rev-Erbα.
Rev-Erbα also plays an important role in maintaining proper timing of the circadian system(Reference Preitner, Damiola and Lopez-Molina28). Thus, in the case of the HF group, the differential response of Bmal1 expression with respect to the other clock genes and clock-controlled genes obtained in the present study could also be related to the expression of Rev-Erbα, as this gene acts as a negative regulator of Bmal1 (Reference Preitner, Damiola and Lopez-Molina28). This situation did not take place in the case of the RSV-treated rats in which both Bmal1 and Rev-Erbα were down-regulated. Consequently, further research is needed to gain more insight into the relationship between Bmal1 and Rev-Erbα.
Although the present study focused on adipose tissue, we also analysed the expression of clock genes and clock-controlled genes in the liver in order to know whether the effects of resveratrol were tissue-dependent. We observed that rats from the RSV group showed a down-regulation of Rev-Erbα in this organ, similar to that observed in the epididymal adipose tissue. This result reinforces the potential use of resveratrol as a molecule affecting clock machinery.
It has been reported that many health benefits associated with resveratrol have been attributed to its ability to mimic the effects of an energy-restrictive diet(Reference Barger, Kayo and Vann19). On the other hand, it has been proposed that fasting or energy restriction could reverse circadian rhythmicity disruption in Rev-Erbα caused by high-fat feeding, by influencing the circadian phase or the mRNA level. In good accordance with these facts, in the present study, resveratrol down-regulated Rev-Erbα, imitating the reported effects of energy restriction.
In conclusion, resveratrol reverses the alteration induced by high-fat feeding in the expression of Rev-Erbα in the adipose tissue, which means that clock machinery is a target for this polyphenol in its protective role on obesity. This change is related to reduced lipogenesis, which might be involved in the body fat-lowering effect of this molecule.
Acknowledgements
The present study was supported by grants from the Instituto de Salud Carlos III (RETIC PREDIMED, CIBERObn), the Spanish Government of Science and Innovation (BFU2011-24720 and AGL2011-27406-ALI), the Tomás Pascual and Pilar Gómez-Cuétara Foundations, the Government of the Basque Country (IT-386-10, IT-572-13), the Séneca Foundation from the Government of Murcia (15123/PI/10) and the University of the Basque Country (UPV/EHU) (ELDUNANOTEK UFI11/32). The contributions of each author were as follows: M. T. M. and N. A. took care of the animals during the experimental period and prepared the experimental diets; J. M. and N. A. performed the RNA quantification by real-time RT-PCR, Western blot analysis and enzyme activity assessment; J. A. M. carried out the bibliography revision; M. G. and M. P. P supervised the results and wrote the manuscript. None of the authors has any conflict of interest to declare.