Introduction
“Ecosystem services” are the benefits that mankind obtains from natural ecosystems (Millennium Ecosystem Assessment 2005, Daily et al. Reference Daily, Polasky, Goldstein, Kareiva, Mooney, Pejchar, Ricketts, Salzman and Shallenberger2009) including food, fresh water and the maintenance of an equable climate. Human activities put pressure on natural systems, and obtaining one benefit (such as fish for food) from an ecosystem may impact its ability to provide other benefits (such as supporting biodiversity). Organizations charged with managing human activities that impact ecosystems must therefore make trade-offs between the different benefits that ecosystems provide (McLeod & Leslie Reference McLeod and Leslie2009, Link Reference Link2010, Reference Watters, Hill, Hinke, Matthews and ReidWatters et al. in press).
Recent “ecosystem assessments” have attempted to collate information on the character, status, distribution and value of ecosystem services at global or regional scales (IPBES 2012). The objective of collating such information is to clarify how ecosystems, the achievement of social and economic goals and the intrinsic value of nature are interconnected (Ash et al. Reference Ash, Blanco, Brown, Garcia, Henrichs, Lucas, Raudsepp-Hearne, Simpson, Scholes, Tomich, Vira and Zurek2010). Such assessments attempt to translate the complexity of nature into functions that can be more readily understood by decision-makers and non-specialists. Their authors suggest that this increases the transparency of trade-offs associated with decisions that may impact ecosystems (Carpenter et al. Reference Carpenter, De Fries, Dietz, Mooney, Polasky, Reid and Scholes2006, Beaumont et al. Reference Beaumont, Austen, Atkins, Burdon, Degraer, Dentinho, Derous, Holm, Horton, van Ierland, Marboe, Starkey, Townsend and Zarzycki2007, Fisher et al. Reference Fisher, Turner and Morling2009, UK NEA 2011).
The continent of Antarctica and the surrounding Southern Ocean have, to date, been under-represented in global ecosystem assessments (e.g. Millennium Ecosystem Assessment 2005, UNEP 2010, 2012) and have not been the subject of any detailed regional assessment. This continent and ocean (which we subsequently refer to as the Antarctic) cover 9.7% of the Earth's surface area and play significant roles in the functioning of the Earth system (Lumpkin & Speer Reference Lumpkin and Speer2007, Mayewski et al. Reference Mayewski, Meredith, Summerhayes, Turner, Worby, Barrett, Casassa, Bertler, Bracegirdle, Naveira Garabato, Bromwich, Campbell, Hamilton, Lyons, Maasch, Aoki, Xiao and van Ommen2009). Their under-representation in ecosystem assessments potentially limits the information available for decision-making about regional and global activities that impact Antarctic ecosystems. It could also lead to underestimates of the consequences of change in Antarctic ecosystems and the global significance of the services they provide.
The governance system for the Antarctic comprises a set of international agreements known as the Antarctic Treaty System (ATS). These treaties imply that the management of activities that impact ecosystems should consider the associated trade-offs. For example, the Protocol on Environmental Protection (1991) recognized “the intrinsic value of Antarctica, including its wilderness and aesthetic values and its value as an area for the conduct of scientific research, in particular research essential to understanding the global environment” (http://www.ats.aq/documents/recatt/Att006_e.pdf, accessed April 2013). Decisions on the conduct of human activities, including scientific research, must therefore consider potential impacts on environmental, aesthetic and wilderness values. The Convention on the Conservation of Antarctic Marine Living Resources underpins the management of fishing activities in the Southern Ocean. The Convention entered into force in 1982, and established the Commission for the Conservation of Antarctic Marine Living Resources as its decision-making body. The acronym ‘CCAMLR’ is often used to refer to both the Convention and the Commission. In this paper, we use ‘CCAMLR’ to refer to the Commission and ‘the Convention’ to refer to the legal instrument. The Convention aims to ensure the “rational use” of marine living resources subject to “principles of conservation” (Fig. 1) including the maintenance of harvested stocks and of ecological relationships between harvested stocks and other species, the recovery of previously depleted stocks, and the prevention of irreversible change (http://www.ccamlr.org/en/document/publications/convention-conservation-antarctic-marine-living-resources, accessed April 2013). Decisions that comply with the Convention must therefore consider the trade-offs between the current benefit of catches, the benefit of future catches from a healthy stock, and the more general benefits of a healthy ecosystem.

Fig. 1 The three-way trade-off used in krill fishery management and its relationship with conservation principles and ecosystem services. The goals of ecosystem-based management (McLeod et al. 2009) map directly onto the principles of conservation set out in the Convention (two left hand columns). The three-way trade-off (yellow boxes) is influenced primarily by the principles of conservation, and it explicitly considers maintenance of provisioning services (fishery catch) in the present (fishery performance) and in the future (status of the krill stock). It also considers the status of predator populations. Ideally krill fishery management should consider fishery impacts on all ecosystem services. The krill stock and predator populations are indicators of ecosystem health, but whether they are useful indicators of other ecosystem services (red lines) is unknown.
The purpose of the current paper is to review existing knowledge of Southern Ocean ecosystem services and the way this knowledge is currently used in decision-making. We collate available information on the identity, distribution, beneficiaries and global significance of Antarctic marine ecosystem services. We use the management of the main Southern Ocean fishery, which harvests Antarctic krill, Euphausia superba Dana, as a case study to explore the extent to which regional decision-making currently uses the type of information that formal ecosystem assessments generate. A full assessment of the status, trends and value of Southern Ocean ecosystem services is beyond the scope of this study, but we discuss the further work required and the potential benefits of conducting a formal ecosystem assessment. While we acknowledge that these objectives are also relevant to the terrestrial Antarctic, we limit our consideration to the marine ecosystem services of the Southern Ocean. For the purposes of this study, we define the Southern Ocean as the area covered by the Convention (http://www.ccamlr.org/en/organisation/convention-area, accessed April 2013). The northern boundary of this area approximates to the position of the Antarctic Polar Front, which is an important ecological boundary between neighbouring oceans. This front is where cold polar surface waters sink beneath temperate surface waters. It is generally located between c. 50°S and 60°S (Moore et al. Reference Moore, Abbott and Richman1997); the higher latitude being the northern boundary of all other ATS agreements (http://www.ats.aq/imagenes/info/antarctica_e.pdf, accessed April 2013).
The following two sections provide brief introductions to ecosystem assessment and direct human interactions with the Southern Ocean ecosystem. Tables I and II present key information about Southern Ocean ecosystem services, and the remaining sections consider the existing use of information on ecosystem services in the management of the Antarctic krill fishery in the Scotia Sea and southern Drake Passage. This forms the basis for our discussion of how an ecosystem assessment might aid CCAMLR's decision-making processes.
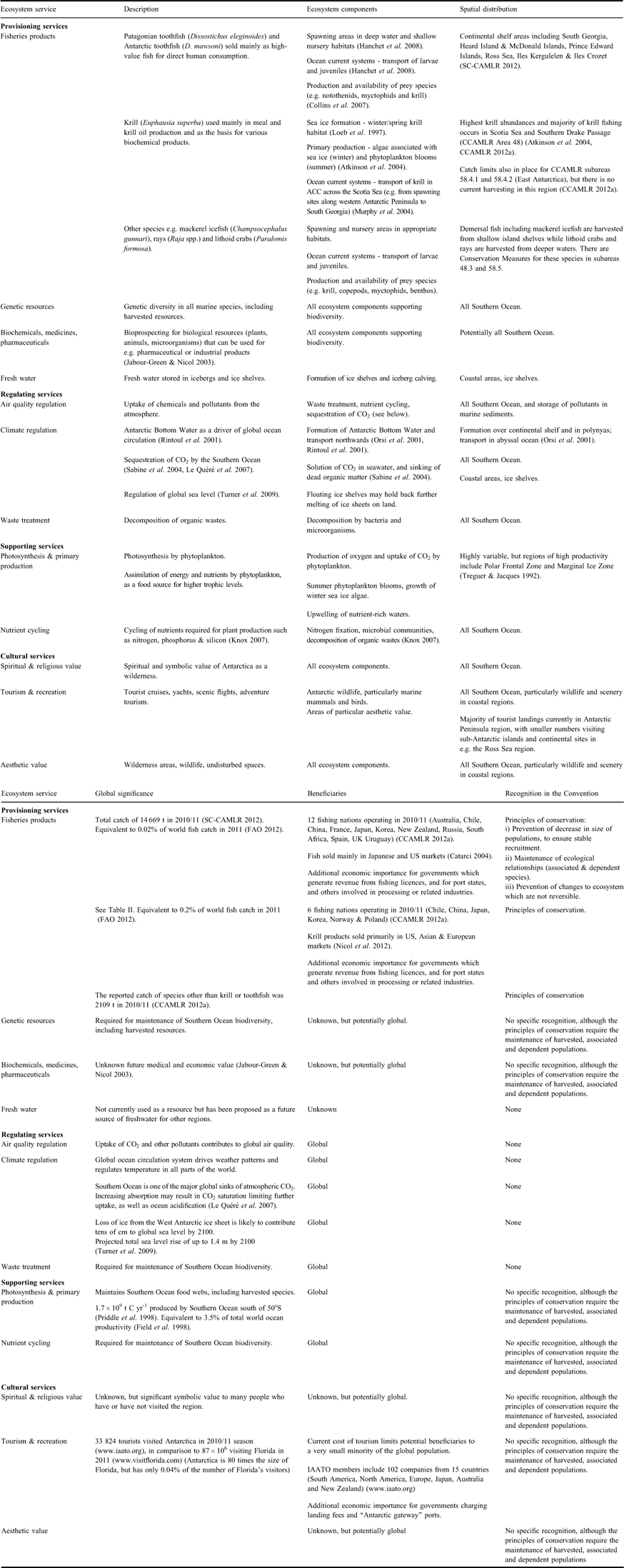
Table I Summary of ecosystem services provided by the Southern Ocean. The “Ecosystem components” column identifies the ecosystem components that are critical to the provision of the relevant service.

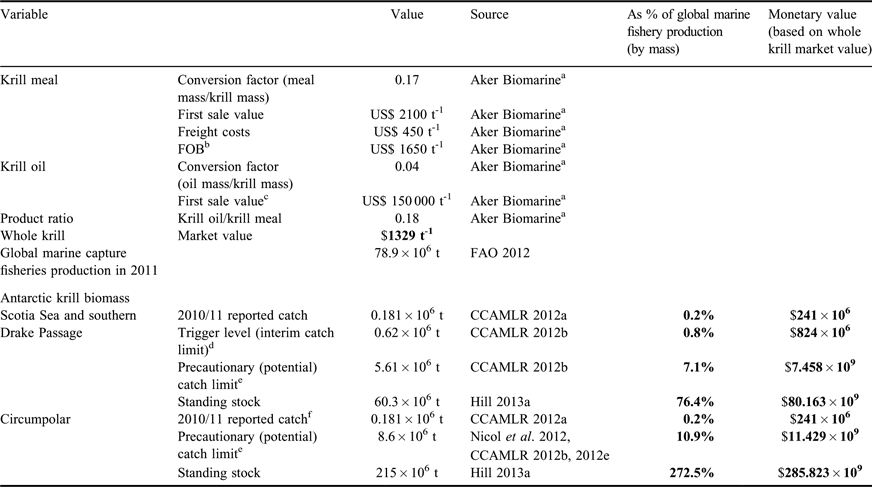
Table II Comparative value of the current catch, catch limits, and standing stock estimates of Antarctic krill at two geographic scales. Values in bold are the results of our calculations, which include values based on market values of krill products and equivalent percentages of global marine capture fishery production (by mass). Other values are the assumptions on which these results are based and were obtained from the stated sources.

aInformation supplied December 2011 by Aker Biomarine, a major krill fishing company.
bFree on board value (FOB) = market value minus freight costs.
cFirst sale value for krill oil does not include production or freight costs.
dThe “trigger level” is the term used in Conservation Measure 51-01 (CCAMLR 2012b) to describe the currently operational catch limit. This limit is in place until a procedure for subdivision of the overall catch limit into smaller management units has been established. We have referred to this as the “interim catch limit” in the main text.
eThe “precautionary catch limit” is the term used in Conservation Measures (CCAMLR 2012b, 2012c) to describe the total catch that could be permitted once spatial subdivision has been agreed.
fAlthough there are catch limits for areas outside the Scotia Sea and southern Drake Passage, there were no reported catches for these areas in 2010/11.
Ecosystem assessment
Ecosystem assessments aim to comprehensively characterize the status and trends of relevant ecosystems, the services they provide, the drivers of change, and the potential consequences of such change (Carpenter et al. Reference Carpenter, De Fries, Dietz, Mooney, Polasky, Reid and Scholes2006, Ash et al. Reference Ash, Blanco, Brown, Garcia, Henrichs, Lucas, Raudsepp-Hearne, Simpson, Scholes, Tomich, Vira and Zurek2010). This includes identifying how ecosystem services affect human well-being, who benefits, and where these beneficiaries are located. It can include identifying the specific value of ecosystem services to their beneficiaries (TEEB 2010). An ecosystem assessment adds value to existing information by clarifying how ecosystems, human well-being and the intrinsic value of nature are interconnected (UK NEA 2011). The practical purpose of these assessments is to provide information that can help decision-makers to better understand how their decisions might change specific ecosystem services. This theoretically equips decision-makers to choose policies that sustain the appropriate suite of services (Ash et al. Reference Ash, Blanco, Brown, Garcia, Henrichs, Lucas, Raudsepp-Hearne, Simpson, Scholes, Tomich, Vira and Zurek2010).
The Millennium Ecosystem Assessment (MA) was a landmark example of a global ecosystem assessment (Millennium Ecosystem Assessment 2005). Its objective was to “assess the consequences of ecosystem change for human well-being”, and it established a framework which has formed the basis for a number of subsequent global and regional ecosystem assessments (e.g. CAFF 2010, UK NEA 2011, UNEP 2012). The MA recognized four categories of ecosystem services: provisioning (e.g. food, freshwater); regulating (e.g. climate regulation, water purification); cultural (e.g. aesthetic benefits and recreation); and supporting (e.g. nutrient cycling and primary production). These categories notably exclude the roles played by polar icecaps in storing water that would otherwise increase sea levels, and by sea ice in holding back continental ice and increasing the Earth's albedo. They also exclude some naturally occurring resources such as minerals and hydrocarbons.
The MA definition of ecosystem services includes benefits that are directly perceived and used by people (such as food and water) and those that are not (such as storm regulation by wetlands) (Costanza Reference Costanza2008). Direct-use benefits of ecosystem services may be consumptive (e.g. the consumption of wild caught fish), or non-consumptive (e.g. the enjoyment of those fish by scuba divers) (Saunders et al. Reference Saunders, Tinch and Hull2010). Non-use benefits may be derived, for example, from the knowledge that a resource or service exists or is being maintained (Ledoux & Turner Reference Ledoux and Turner2002, Saunders et al. Reference Saunders, Tinch and Hull2010). Benefits may be enjoyed at the location of a particular ecosystem service (e.g. local subsistence fishing) or at a great distance from it (e.g. large-scale commercial fishing by far seas fleets with global markets).
By definition, ecosystem services have value to their beneficiaries. Ecosystem assessments aim to identify the relative value of each ecosystem service based on various measures. In the case of consumptive use, it might be possible to measure value in economic terms, but it is also important to consider other types of value (Costanza et al. Reference Costanza, D'Arge, De Groot, Farber, Grasso, Hannon, Limburg, Naeem, O'Neill, Paruelo, Raskin, Sutton and van den Belt1997). Various authors have described non-use benefits in terms of existence or presence value, altruistic value (knowledge of benefits being used by the current generation), and bequest value (knowledge of benefits being used by future generations) (Gilpin Reference Gilpin2000, Chee et al. Reference Chee2004, Saunders et al. Reference Saunders, Tinch and Hull2010). The preservation of a resource or service for future use, or the avoidance of irreversible decisions until further information is available (Millennium Ecosystem Assessment 2005) is sometimes considered as a use value in itself (Saunders et al. Reference Saunders, Tinch and Hull2010). However, it may be categorised separately as an unknown use, including a ‘quasi-option value’ where future use assumes the availability of increased knowledge or technology (Ledoux & Turner Reference Ledoux and Turner2002, Chee et al. Reference Chee2004).
The objective of ecosystem assessment to provide a comparison between ecosystem services has led to attempts to express these different values in standardized, and often monetary, terms. The monetary value of an ecosystem service is arguably equivalent to the cost of replacing that service or finding another means of gaining similar benefits (Ledoux & Turner Reference Ledoux and Turner2002). In some cases, particularly for those services which constitute the Earth's life support systems (e.g. climate regulation) this value is unlimited, because the service would be irreplaceable if lost completely.
The Total Economic Value (TEV) framework is increasingly used to assess the value of ecosystem services by combining both monetary and non-monetary aspects of overall value (Ledoux & Turner Reference Ledoux and Turner2002). Figure 2 sets out a simple TEV framework adapted from previous studies (Ledoux & Turner Reference Ledoux and Turner2002, Chee et al. Reference Chee2004, Saunders et al. Reference Saunders, Tinch and Hull2010). The loss of ‘natural capital’ such as forests or fish stocks is not included in traditional economic accounting models such as Gross Domestic Product (GDP) (Dasgupta Reference Dasgupta2010). In some cases, the exploitation of natural resources might result in a positive growth in GDP, when the degradation or unsustainable use of those resources has in fact reduced natural capital. Valuation of ecosystem services provides information that might help to inform policy decisions that reduce such loss or degradation of natural capital (Costanza et al. Reference Costanza, D'Arge, De Groot, Farber, Grasso, Hannon, Limburg, Naeem, O'Neill, Paruelo, Raskin, Sutton and van den Belt1997, Ledoux & Turner Reference Ledoux and Turner2002).

Fig. 2 The Total Economic Value (TEV) framework for valuation of ecosystem services (adapted from Ledoux & Turner Reference Ledoux and Turner2002, Chee et al. Reference Chee2004, Saunders et al. Reference Saunders, Tinch and Hull2010).
Human uses of the Southern Ocean
The Southern Ocean is the only ocean that does not border a permanently inhabited landmass and, consequently, it was unknown and unexploited until the late 1700s. The economic importance of its ecological resources grew rapidly following Captain Cook's discovery of abundant fur seals at South Georgia in 1775. The Southern Ocean became the world's main source of seal products in the 1800s and whale products in the 1900s (Bonner Reference Bonner1984, Headland Reference Headland1992). Populations of fur seals were reduced almost to extinction by the early 19th century. Attention then shifted to elephant seals and southern right whales. By the first half of the 20th century, these stocks had also declined and improved technology allowed offshore hunting of other baleen whales and sperm whales to become established. Whaling ceased in the 1960s when it was no longer economically viable. Finfish and then Antarctic krill became the major focus for exploitation, which continues until the present-day. Historical harvesting operations and catch sizes are mainly well documented (e.g. Laws Reference Laws1953, Kock Reference Kock1992, CCAMLR 2012a, Hill Reference Hill2013a, fig 14.5), although illegal, unregulated and unreported (IUU) fishing has occurred, most recently for high-value toothfish (Österblom & Bodin Reference Österblom and Bodin2012). The extent and scale of this living resource extraction, and the fact that some whale and finfish stocks remain depleted (Bonner Reference Bonner1984, Kock Reference Kock1992) demonstrates that the Southern Ocean is far from being a pristine wilderness as it is sometimes characterized.
The hostile and remote nature of the Southern Ocean, and the lack of a permanent human population have constrained direct use of its ecosystem services. Nevertheless, marine harvesting, science and tourism all directly impact the Antarctic environment (Clarke & Harris Reference Clarke and Harris2003, Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey, Moreno and Pfeiffer2009). Scientific research and its associated logistic and support requirements have been a major focus of human activities in Antarctica and the Southern Ocean since the early 20th century. Up to 6000 scientific and support personnel are stationed in and around Antarctica at the peak of the summer season (Clarke & Harris Reference Clarke and Harris2003), and the Antarctic Treaty aims to maintain a high level of protection for the Antarctic environment as a scientific resource. The iconic wildlife, unique seascapes and coastlines, and relative isolation are all important factors in attracting recreational visitors. Antarctic tourism did not become established until the 1970s, and although it has expanded and diversified significantly during the last 40 years the number of visitors remains relatively low (around 35 000 each year; http://iaato.org/tourism-statistics, accessed April 2013).
Ecosystem services provided by the Southern Ocean
Using the four categories identified by the MA, we have identified and described the ecosystem services provided by the Southern Ocean and the ecosystem components corresponding to the provision of these services (Table I). Of the 24 ecosystem services examined by the MA we suggest that 12 have direct relevance in the Southern Ocean. Others are relevant only to terrestrial habitats or where there is a resident human population. Table I also lists the current beneficiaries of each identified ecosystem service and the spatial distribution of these services where applicable. Species that are particularly important to the provision of ecosystem services include harvested species such as Antarctic krill, toothfish, and other fish species; iconic or flagship species (Zacharias & Roff Reference Zacharias and Roff2001) such as penguins, whales, seals and albatrosses; and phytoplankton, zooplankton, and macro-zooplankton species which play key roles in primary production and nutrient cycling. There are potential benefits from services which are as yet unknown in the Southern Ocean. Endemism is high in many marine taxa (Arntz et al. Reference Arntz, Gutt and Klages1997) suggesting the potential for products that cannot be sourced elsewhere. A few genetic and biochemical materials have been patented for use in pharmaceutical or industrial products but the potential of such resources has yet to be fulfilled (Jabour-Green & Nicol Reference Jabour-Green and Nicol2003). Other services such as the provision of freshwater may not be viable or utilized at present, but remain potentially important for the future if there are changes to global supply and demand.
Ecosystem services provided by the Southern Ocean have few direct, local beneficiaries. The provisioning services support consumption elsewhere. For example, markets for toothfish and Antarctic krill products are predominantly in northern hemisphere nations in East Asia, North America, and Europe (Catarci Reference Catarci2004, Nicol et al. Reference Nicol, Foster and Kawaguchi2012). Regulating and supporting services such as climate regulation, ocean circulation and nutrient cycling provide benefits to human populations globally.
Marine ecosystem services may occur within well-defined locations (e.g. the spawning grounds of a particular fish species which support a provisioning service), or across much larger and spatially less distinct areas (e.g. sequestration of CO2 across the entire Southern Ocean). There is some potential for spatially explicit mapping of ecosystem services in the Southern Ocean, for example to illustrate the spatial dimension of catch value (UK NEA 2011). Information is also available on tourist landing sites (http://iaato.org/tourism-statistics) and ship traffic (Lynch et al. Reference Lynch, Crosbie, Fagan and Naveen2010). Mapping of regulating and supporting services may be more difficult to achieve, although datasets such as sea surface chlorophyll concentrations (e.g. http://oceancolor.gsfc.nasa.gov) may serve as useful proxies.
Table II presents some simple estimates of the comparative value of the Antarctic krill stock as an illustration of the value of Southern Ocean ecosystem services. The Antarctic krill stock in the Scotia Sea and southern Drake Passage is managed with an interim catch limit but there is also a higher potential limit, known as the “precautionary catch limit” (CCAMLR 2012b). These two catch limits are respectively equivalent to 0.8% and 7.1% of global marine capture fisheries production in 2011 (FAO 2012) with first sale values of about US$ 824 × 106 yr-1 and US$ 7.4 × 109 yr-1. The comparable first sale value of the global fish catch is c. US$ 85 × 109 yr-1 (Pikitch et al. Reference Pikitch, Rountos, Essington, Santora, Pauly, Watson, Sumaila, Boersma, Boyd, Conover, Cury, Heppell, Houde, Mangel, Plagányi, Sainsbury, Steneck, Geers, Gownaris and Munch2012). The current market for krill oil alone is c. US$ 82 × 106 yr-1 (Hill Reference Hill2013a). These economic values should be considered alongside the value of other ecosystem services provided by the Antarctic krill stock. Pikitch et al. (Reference Pikitch, Rountos, Essington, Santora, Pauly, Watson, Sumaila, Boersma, Boyd, Conover, Cury, Heppell, Houde, Mangel, Plagányi, Sainsbury, Steneck, Geers, Gownaris and Munch2012) estimated that the contribution to predator production made by Antarctic krill is higher than that of any comparable species in the world's oceans. Other types of value based on the components of TEV (Fig. 2) might include option, existence, or bequest value. Investment in research and conservation gives some indication of the importance society currently attaches to ecological resources. The coverage of closed or protected areas which limit fishery access, for example at the South Orkney Islands (CCAMLR 2012c) and South Georgia (http://www.sgisland.gs/download/MPA/MPA%20Plan%20v1-1.01%20Feb%2027_12.pdf), is a non-monetary indication of conservation investment. However, the cost of research and protection is likely to be much lower than the hypothetical replacement value.
Existing use of information about ecosystem services in the ATS
Ecosystem assessments aim to characterize ecosystem services in terms of their identity and status. This status might be assessed relative to reference points defining desirable states. Ecosystem assessments also attempt to identify the beneficiaries of ecosystem services and to evaluate potential drivers and consequences of future ecosystem change. This is intended to facilitate decision-making based on trade-offs between ecosystem services. This section uses the Antarctic krill fishery in the Scotia Sea and southern Drake Passage as a case study to identify the extent to which management processes consider trade-offs and use the types of information that are collated in ecosystem assessments.
Overview of decision making within CCAMLR
The instruments of the ATS govern existing and potential human activities in the Southern Ocean, although these instruments are legally binding only on signatory nations. The Protocol on Environmental Protection prohibits mineral exploitation south of 60°S and specifies the conduct of scientific, logistic and tourist operations. CCAMLR manages fishing activities in the wider Southern Ocean ecosystem. A total of 8% of this area falls under the jurisdiction of national governments (including the marine areas around Heard Island and McDonald Island, Iles Kerguelen and Iles Crozet, the Prince Edward Islands, South Georgia and the South Sandwich Islands), some of which apply CCAMLR management measures.
CCAMLR manages fishing and related activities by implementing regulations known as Conservation Measures. Commissioners are representatives of national governments. CCAMLR is advised by a Scientific Committee which, in turn, is advised by a number of scientific working groups. Decision-making at each of these levels is by consensus (Hill Reference Hill2013a, fig 14.4).
The Antarctic krill fishery in the Scotia Sea and southern Drake Passage accounted for 91% by mass of the total Southern Ocean catch in the 2010–11 fishing season (CCAMLR 2012a). There are a number of reviews that describe the development of CCAMLR's management approach for this fishery (Constable et al. Reference Constable, De la Mare, Agnew, Everson and Miller2000, Miller & Agnew Reference Miller and Agnew2000, Hill Reference Hill2013a), which we also summarize here.
The Convention's principles of conservation (CCAMLR 1982) were an early articulation of the goals of Ecosystem Based Management. Ecosystem Based Management takes account of trade-offs between ecosystem services, and has the goals of maintaining the ecosystem productivity, health and resilience that underpins the provision of ecosystem services (McLeod & Leslie Reference McLeod and Leslie2009). Management of Antarctic krill fisheries has generally focused on the three-way trade-off between the performance of the fishery, the status of the krill stock, and the status of selected krill predators. In this trade-off, the status of krill predators is used as a proxy for the health and resilience of the wider ecosystem (Fig. 1), although CCAMLR has also considered other impacts of the fishery, such as larval fish bycatch (Agnew et al. Reference Agnew, Grove, Peatman, Burn and Edwards2010).
The Antarctic krill harvest from the Scotia Sea and southern Drake Passage has been capped at 620 000 t yr-1 since CCAMLR first began to regulate the fishery in 1991. This interim catch limit is less than the “precautionary catch limit” (currently 5.61 × 106 t yr-1) which has been updated a number of times in response to revised estimates of Antarctic krill biomass (e.g. Trathan et al. Reference Trathan, Everson, Miller, Watkins and Murphy1995, Hewitt et al. Reference Hewitt, Watkins, Naganobu, Sushin, Brierley, Demer, Kasatkina, Takao, Goss, Malyshko, Brandon, Kawaguchi, Siegel, Trathan, Emery, Everson and Miller2004a, SC-CAMLR 2010). The “precautionary catch limit” defines the potential maximum harvest when the management approach is sufficiently developed to allow the interim limit to be removed.
CCAMLR's scientific working groups have used the three-way trade-off to develop and evaluate management approaches that address two key questions: what is the appropriate overall catch limit, and how should this be spatially distributed to minimize local depletion of krill and its predators? The first question led to a set of decision rules which CCAMLR established in the early 1990s to identify the “precautionary catch limit” (SC-CAMLR 1994). These decision rules were formulated for use with simulation models and an estimate of the initial biomass of Antarctic krill, which is assumed to represent the biomass prior to any impacts of fishing. One rule allows for the simulated Antarctic krill stock to be depleted to 75% of its initial biomass. This compares with the maximum sustainable yield reference point which is widely used in other fisheries and allows depletion to around 60% (Smith et al. Reference Smith, Brown, Bulman, Fulton, Johnson, Kaplan, Lozano-Montes, Mackinson, Marzloff, Shannon, Shin and Tam2011). Thus the decision rule reserves a proportion of Antarctic krill production for its predators. Smith et al. (Reference Smith, Brown, Bulman, Fulton, Johnson, Kaplan, Lozano-Montes, Mackinson, Marzloff, Shannon, Shin and Tam2011) suggested that depletion to 75% of initial biomass represents a reasonable trade-off between the benefits of harvesting and ecosystem health. Another rule constrains the risk of the simulated krill population falling to low levels likely to impact productivity.
Work is ongoing within CCAMLR's scientific working groups to address the second question. These groups have identified ecologically-based spatial subdivisions of the fishery (Hewitt et al. Reference Hewitt, Watters, Trathan, Croxall, Goebel, Ramm, Reid, Trivelpiece and Watkins2004b) and assessed the potential consequences of different spatial fishing patterns (Plagányi & Butterworth Reference Plagányi and Butterworth2012, Hill Reference Hill2013b, Reference Watters, Hill, Hinke, Matthews and ReidWatters et al. in press). The krill biomass in any area varies naturally over time (Brierley et al. Reference Brierley, Goss, Grant, Watkins, Reid, Belchier and Robst2002, Atkinson et al. Reference Atkinson, Siegel, Pakhomov and Rothery2004). The patterns of variability are also likely to change in response to climate change and fishing (Everson et al. Reference Everson, Stonehouse, Drewry and Barker1992). It might therefore be appropriate to vary area-specific catch limits, or other activities, such as monitoring, in response to information about the state of the krill stock or the wider ecosystem (Constable Reference Constable2002, Trathan & Agnew Reference Trathan and Agnew2010, SC-CAMLR 2011). CCAMLR's scientific working groups aim to develop a “feedback management procedure” (SC-CAMLR 2011) to address these issues. They have considered the use of data from the fishery, small-scale krill surveys (e.g. Brierley et al. Reference Brierley, Goss, Grant, Watkins, Reid, Belchier and Robst2002) and krill predators (Constable Reference Constable2002, Hill et al. Reference Hill, Forcada, Trathan and Waluda2010) to indicate the state of the ecosystem. However, further work is required on all aspects of the proposed procedure, including definition of its specific objectives.
CCAMLR has not, to date, agreed a management approach that will prevent excessive localized depletion of the krill stock, and consequent impacts on krill predators, if catches increase beyond the interim catch limit. It therefore retains the interim limit and has recently established additional caps within the fishery's four subareas (CCAMLR 2012d).
The Antarctic krill catch increased from 126 000 t in 2001/02 to 181 000 t in 2010/11. This expansion coincided with new developments in harvesting and processing technology and new markets for krill products (Nicol et al. Reference Nicol, Foster and Kawaguchi2012, CCAMLR 2012a). Catches remain below 0.4% of the estimated available biomass in the Scotia Sea and southern Drake Passage (60.3 x 106 t), while the interim catch limit is around 1% of this estimate. These values are low compared with most established fisheries elsewhere in the world (FAO 2012) and compared to the standard reference points used to evaluate sustainability (Worm et al. Reference Worm, Hilborn, Baum, Branch, Collie, Costello, Fogarty, Fulton, Hutchings, Jennings, Jensen, Lotze, Mace, McClanahan, Minto, Palumbi, Parma, Ricard, Rosenberg, Watson and Zeller2009) but some authors have questioned whether any krill fishing is sustainable (Jacquet et al. Reference Jacquet, Pauly, Ainley, Dayton, Holt and Jackson2010).
The decision rules represent a practical solution to the need to balance effects on different ecosystem components, which did not require an economic valuation of the relevant ecosystem services. However, CCAMLR has not yet identified an approach which balances these effects at the appropriate ecological scale, and so relies on interim management measures. The current challenges facing the managers of the krill fishery include increasing demand for krill products, public interest in other ecosystem services that krill may support, and the pressure of climate change. CCAMLR is attempting to meet these challenges through developing a “feedback management procedure”.
Consideration of the character and status of ecosystem services
Antarctic krill is an important species in much of the Southern Ocean, where it is a major prey item for a diverse community of predators including fish, seabirds, marine mammals and cephalopods (Atkinson et al. Reference Atkinson, Siegel, Pakhomov, Jessopp and Loeb2009, Hill et al. Reference Hill, Keeble, Atkinson and Murphy2012). Ecosystem components of interest to CCAMLR therefore include the Antarctic krill stock and its predators. CCAMLR and the wider research community are actively addressing questions about the status and trends of these components. CCAMLR's ecosystem monitoring programme (CEMP) was established in 1987. It aims to detect and record significant changes in critical components of the marine ecosystem and to distinguish between changes due to harvesting of commercial species and changes due to environmental variability, both physical and biological (Croxall Reference Croxall2006). CEMP monitors Antarctic krill and nine predator species (penguins, albatrosses and fur seals) representing the ‘dependent and related populations’ referred to in the Convention's principles of conservation (Fig. 1). The monitored ecosystem components are consistent with the three-way trade-off. The choice of monitored components therefore reinforces the assumption that krill predators are suitable indicators of the wider state of the ecosystem. The spatial scales and species for which the state of predator populations should be evaluated to inform krill fishery management remain to be defined.
In 2000, CCAMLR conducted a multi-national large-scale synoptic survey to estimate the biomass of Antarctic krill in 2 x 106 km2 of the Scotia Sea and southern Drake Passage (Hewitt et al. Reference Hewitt, Watkins, Naganobu, Sushin, Brierley, Demer, Kasatkina, Takao, Goss, Malyshko, Brandon, Kawaguchi, Siegel, Trathan, Emery, Everson and Miller2004a). Some CCAMLR Members also monitor krill biomass in smaller areas. For example, the UK has estimated biomass in an area of at least 8000 km2 to the north of South Georgia since 1981 and on a regular basis since 1996 (Brierley et al. Reference Brierley, Goss, Grant, Watkins, Reid, Belchier and Robst2002). A series of studies that integrate data from national science programmes has, independently of CCAMLR, produced recent estimates of circumpolar krill biomass and production, and an assessment of trends in krill abundance (Atkinson et al. Reference Atkinson, Siegel, Pakhomov and Rothery2004, Reference Atkinson, Siegel, Pakhomov, Jessopp and Loeb2009). Other studies, mainly associated with CEMP data, have assessed the status and trends of various krill predator populations (e.g. Forcada et al. Reference Forcada, Trathan, Reid and Murphy2005, Forcada & Trathan Reference Forcada and Trathan2009). Turner et al.'s (Reference Turner, Bindschadler, Convey, Di Prisco, Fahrbach, Gutt, Hodgson, Mayewski and Summerhayes2009) review of Antarctic climate change and environment collated much of the relevant information from published scientific studies, while Flores et al. (Reference Flores, Atkinson, Kawagushi, Krafft, Milinevsky, Nicol, Reiss, Tarling, Werner, Bravo Rebolledo, Cirelli, Cuzin-Roudy, Fielding, Groeneveld, Haraldsson, Lombana, Marschoff, Meyer, Pakhomov, Rombolá, Schmidt, Siegel, Teschke, Tonkes, Toullec, Trathan, Tremblay, van de Putte, van Franeker and Werner2012) provided a more krill-focused review.
Many national science programmes and several international science coordination and implementation bodies have a Southern Ocean focus, addressing questions about the status and trends of ecosystems (e.g. Murphy et al. Reference Murphy, Cavanagh, Hofmann, Hill, Constable, Costa and Doney2012). These programmes have sometimes identified a particular ecosystem service, or the need to manage activities that affect ecosystem services, as the motivation or benefit of their research, but none has aimed to provide a comprehensive assessment of ecosystem status and trends.
Definitions of the desirable states of ecosystem components and of the fishery (and therefore undesirable states to avoid) remain elusive (Hill Reference Hill2013b). Two prominent recent studies have suggested tentative reference points for “forage” species, such as krill, that support diverse predators. Cury et al. (Reference Cury, Boyd, Bonhommeau, Anker-Nilssen, Crawford, Furness and Sydeman2011) analysed the relationship between prey availability and seabird breeding success. They recommended maintaining forage species above a third of the maximum biomass observed in long-term studies. Smith et al. (Reference Smith, Brown, Bulman, Fulton, Johnson, Kaplan, Lozano-Montes, Mackinson, Marzloff, Shannon, Shin and Tam2011) used ecosystem models to assess the propagation of fishery impacts through the foodweb. They suggested maintaining forage species above 75% of their unexploited biomass. Each of these reference points carries caveats which will need to be addressed before implementation. The Cury et al. (Reference Cury, Boyd, Bonhommeau, Anker-Nilssen, Crawford, Furness and Sydeman2011) analysis was based on aggregated data from a range of ecosystems, including the Scotia Sea. Simplistic application of its recommendations to the krill fishery suggests that krill should be maintained at levels which were only observed in six of the 21 years analysed. This highlights the difficulties in practical application of universal reference points. More detailed consideration of the scale of predator foraging, the response of different predators, and the current state of the ecosystem will be necessary to develop recommendations for the krill fishery. The 75% reference point has already been used to suggest overall krill catch limits, but CCAMLR recognizes that by itself this does not provide adequate protection against localized depletion of krill and consequent impacts on predators (Hewitt et al. Reference Hewitt, Watters, Trathan, Croxall, Goebel, Ramm, Reid, Trivelpiece and Watkins2004b).
Consideration of beneficiaries of ecosystem services
The Preamble to the Antarctic Treaty (1959) recognized that peaceful use of the Antarctic and scientific cooperation are in the interests of “all mankind” (http://www.ats.aq/documents/ats/treaty_original.pdf, accessed April 2013). The Convention states a commitment to “rational use”, which is often interpreted by CCAMLR Members as meaning sustainable fishing. However, the Convention does not explicitly define the term, meaning that it can be applied to the use of other ecosystem services (Reference Watters, Hill, Hinke, Matthews and ReidWatters et al. in press).
Questions about the ability of ecosystem services to supply local needs are inappropriate for the Southern Ocean due to the geographical separation between these ecosystem services and their beneficiaries. This fact might partly explain why there has been little direct consideration within CCAMLR of the relationships between ecosystem services and human well being.
The fishing industry and its employees, suppliers and customers are direct beneficiaries of the Antarctic krill fishery. The beneficiaries of other ecosystem services that the fishery could impact are less clearly defined, although these could include tourists, scientists, and others who might benefit from the maintenance of predator populations and the wider ecosystem (see Table I). The consensus decision-making in CCAMLR provides a mechanism for accommodating multiple opinions representing multiple ways of valuing different ecosystem services. However, consensus decision-making also has recognized drawbacks including the disproportionate influence of minority opinions and a tendency to default to the status quo. For many Members there will be pressure to ensure that decisions are defensible in terms of both the Convention and public opinion. Nonetheless, in order to have an influence, opinions must be represented at national government level, and there is no automatic requirement to represent all beneficiaries, or to consider the relative value of different ecosystem services to different beneficiaries.
Several conservation-focused non-governmental organisations (NGOs) also take an interest in krill fishery issues. Some of these have observer status within CCAMLR under the umbrella of the Antarctic and Southern Ocean Coalition. However, few interest groups or direct beneficiaries have stated their specific objectives for krill fishery management. Hill (Reference Hill2013a) noted that most groups identify “sustainability” as a key requirement but that few have provided a tangible definition of this term. Furthermore, some uses of this term are mutually contradictory. Nonetheless, Österblom & Bodin (Reference Österblom and Bodin2012) reported that 117 diverse organizations responded to the crisis of IUU harvesting of toothfish in the Southern Ocean with shared purpose. Their actions resulted in a substantial reduction in IUU fishing. This suggests that effective cooperation between diverse interest groups is possible.
CCAMLR faces the challenge of making operational decisions on the basis of its conservation principles that are acceptable to a diverse community of beneficiaries and interest groups. At present there is little information about the values that these groups place on ecosystem services, or their specific objectives for the ecosystem or the fishery. The types of question posed by ecosystem assessments might help to identify these values and objectives.
Consideration of future change
The MA examined how ecosystems and the services they provide might change under plausible future scenarios. This is a key question being asked by many Antarctic-focused national science programmes and international coordinating bodies including the Scientific Committee on Antarctic Research and the Integrating Climate and Ecosystem Dynamics in the Southern Ocean programme (Murphy et al. Reference Murphy, Cavanagh, Hofmann, Hill, Constable, Costa and Doney2012), in conjunction with ATS bodies including CCAMLR. The Intergovernmental Panel on Climate Change intends to increase its coverage of the status and prognosis for Southern Ocean ecosystems with a dedicated chapter in the forthcoming Fifth Assessment Report. The impetus for such activity has come mainly from the scientific community but the strong interaction between scientists and decision makers within CCAMLR ensures shared purpose.
The paucity of historical data presents a particular challenge for defining baseline status and relative reference points for living components of the Southern Ocean ecosystem (Hill et al. Reference Hill, Murphy, Reid, Trathan and Constable2006, Trathan et al. Reference Trathan, Ratcliffe and Masden2012). Clarke & Harris (Reference Clarke and Harris2003) and Turner et al. (Reference Turner, Bindschadler, Convey, Di Prisco, Fahrbach, Gutt, Hodgson, Mayewski and Summerhayes2009) identified key influences on the current status of Antarctic ecosystems, and suggest potential ecosystem responses to further change. Climate forcing is a major influence on the Southern Ocean ecosystem (Everson et al. Reference Everson, Stonehouse, Drewry and Barker1992, Turner et al. Reference Turner, Bindschadler, Convey, Di Prisco, Fahrbach, Gutt, Hodgson, Mayewski and Summerhayes2009). This apparently results from complex interactions between natural climate processes, and the anthropogenic effects of the ozone hole and greenhouse gases (Turner et al. Reference Turner, Bindschadler, Convey, Di Prisco, Fahrbach, Gutt, Hodgson, Mayewski and Summerhayes2009, Turner & Overland Reference Turner and Overland2009). Although limited human activity in the Southern Ocean constrains the potential direct influences (Trathan & Agnew Reference Trathan and Agnew2010), potentially important drivers of change include: fishing; the ongoing consequences of historical exploitation of seals, whales and fish; pollution; disease; and invasive species (Clarke & Harris Reference Clarke and Harris2003, Trathan & Reid Reference Trathan and Reid2009).
The Convention identifies the importance of the effects of fishing and associated activities “on the marine ecosystem and of the effects of environmental changes”. CCAMLR's 2009 resolution 30/XXVIII (http://www.ccamlr.org/en/resolution-30/xxviii-2009, accessed April 2013) also recognized the importance of climate change, urging “increased consideration of climate change impacts in the Southern Ocean to better inform CCAMLR management decisions” and encouraging “an effective global response to address the challenge of climate change”. These statements require ongoing consideration of how to secure the delivery of a limited set of ecosystem services while minimizing the impact on others. Further work remains necessary to quantify and forecast environmental change, to understand levels of uncertainty, and to assess potential impacts on ecosystem services, including their social and economic implications.
Discussion
The previous sections have provided a preliminary characterization of the Southern Ocean's ecosystem services, demonstrating their global importance in terms of climate regulation, food supply and the maintenance of biodiversity. The high estimated value of the Antarctic krill stock relative to global fishery landings provides an illustration of this global significance. We have also discussed the extent to which the functions of ecosystem assessment are already integrated into the management of the Antarctic krill fishery. This demonstrates that trade-offs between the benefits obtained from harvesting and the potential impacts on other ecosystem services are a major component of CCAMLR's decision-making process.
The governance system for the Southern Ocean offers unique opportunities for managing the trade-offs between ecosystem services because its influence covers a whole ocean ecosystem. In 2009, CCAMLR designated a Marine Protected Area located entirely within the High Seas (CCAMLR 2012c). This global first is an important milestone in protecting ecosystems that are beyond national jurisdiction. Furthermore the Convention's principles of conservation effectively require management that accounts for such trade-offs. The developing management of the Antarctic krill fishery acknowledges these trade-offs, but simplifies them to a three-way consideration of fishery performance and the status of krill and predator populations. It is appropriate to assess whether this three-way trade-off fully represents CCAMLR's responsibilities under the Convention and the wider ATS. CCAMLR faces further challenges in developing its management approach, and in ensuring that this approach is co-ordinated with organizations responsible for other human activities at both the global and regional scale.
The ecosystem services of the Southern Ocean are a global resource from which all of mankind indirectly benefits. Most beneficiaries of these ecosystem services never have any direct contact with the ecosystem. There is, however, a small and relatively privileged group of direct beneficiaries that includes fishing and tourism companies, affluent tourists and consumers of the premium products (such as krill oil and Antarctic toothfish) derived from Antarctic fisheries. These activities also create employment and therefore another category of beneficiary. In their consideration of growing demand for marine fisheries products, Garcia & Rosenburg (Reference Garcia and Rosenburg2010) identified krill as a resource that could perhaps support further exploitation. Thus, the composition of the group of direct beneficiaries could change over time. The spatial disconnect between the ecosystem services and the majority of beneficiaries means that the role of interest groups as intermediaries between beneficiaries and managers is particularly pronounced. There is an important distinction between beneficiaries and interest groups. Beneficiaries include the whole human race benefiting from a wide range of ecosystem services, while interest groups often focus on a narrow set of benefits and objectives. The specific requirements of beneficiaries are not currently well understood with the consequence that CCAMLR is yet to define operational objectives for the state of the krill stock, its predators and the wider ecosystem (Hill Reference Hill2013a, Reference Hill2013b, Reference Watters, Hill, Hinke, Matthews and ReidWatters et al. in press).
The Southern Ocean ecosystem is strongly influenced by human activities elsewhere (Clarke & Harris Reference Clarke and Harris2003), and is particularly vulnerable to the effects of climate change (Turner et al. Reference Turner, Bindschadler, Convey, Di Prisco, Fahrbach, Gutt, Hodgson, Mayewski and Summerhayes2009). Ecosystem managers arguably have a duty to maintain the regulatory and supporting services required for healthy ecosystems, and therefore to ensure appropriate interaction with the wider global community on such issues. Identifying objectives that are consistent with its responsibility and influence are an additional challenge faced by CCAMLR.
Ecosystem assessment could help CCAMLR to meet these various challenges by providing a comprehensive characterization of the status, trends, and drivers of change to ecosystems and the services they provide for human well-being. A regional ecosystem assessment for the Southern Ocean would address its under-representation in existing global assessments. Such an assessment would also have benefits for CCAMLR and the wider ATS. Firstly, it would increase knowledge about the connections between the broad suite of Southern Ocean ecosystem services and the social and economic goals of CCAMLR Members. Clearer information on the value of ecosystem services would address the existing need for information about the objectives for each component of the three-way trade-off. It would also promote consideration of ecosystem services that are not currently represented in decision-making. Secondly, an assessment which gives equal consideration to the full range of provisioning, supporting, regulating and cultural services would be a substantial undertaking involving a wide community. This, in itself, could help forge more substantial links between the different components of the ATS. The end product would provide a consistent basis for coordinating activities related to managing or understanding ecosystem impacts.
The information presented here could provide a starting point for such an assessment. New research would be needed to fill some obvious gaps such as the spatial mapping (e.g. Naidoo et al. Reference Naidoo, Balmford, Costanza, Fisher, Green, Lehner, Malcolm and Ricketts2008, Maes et al. Reference Maes, Braat, Jax, Hutchins, Furman, Termansen, Luque, Paracchini, Chauvin, Williams, Volk, Lautenbach, Kopperoinen, Schelhaas, Weinert, Goosen, Dumont, Strauch, Gorg, Dormann, Katwinkel, Zulian, Varjopuro, Ratamaki, Hauck, Forsius, Hengeveld, Perez-Soba, Bouraoui, Scholz, Schulz-Zunkel, Lepisto, Polishchuck and Bidoglio2011) and economic valuation (e.g. Costanza et al. Reference Costanza, D'Arge, De Groot, Farber, Grasso, Hannon, Limburg, Naeem, O'Neill, Paruelo, Raskin, Sutton and van den Belt1997) of ecosystem services, and the assessment would serve as a gap analysis to highlight other data needs. Best-practice developed in many other regional assessments could be useful (Ash 2010). CCAMLR is a user of information on the status and trends of marine ecosystems but it does not fund or directly mandate the collection of such data. The reliance of CCAMLR on donated information is a significant challenge to both the achievement of an ecosystem assessment and the long-term management of ecosystem services in the Southern Ocean (Hill Reference Hill2013a, Reference Hill2013b). There are several potential solutions, including a new initiative by the fishing industry to support the scientific work of CCAMLR (Nicol et al. Reference Nicol, Foster and Kawaguchi2012). We acknowledge that an ecosystem assessment would be a significant task in terms of resource requirements and coordination effort, but we believe it would deliver significant and long-term practical benefits.
Conclusion
The ecosystem services provided by the Southern Ocean are significant on a global scale, as illustrated by the potential of Antarctic krill to supply the equivalent of 11% of current world fishery landings. The terms “ecosystem services” and “ecosystem assessment” are not commonly used within the community concerned with managing human activities in the Southern Ocean. Nonetheless this community is actively gathering and applying much of the information that ecosystem assessments seek to collate. The Convention, in particular, articulates the requirement to consider trade-offs between ecosystem services. The management of the krill fishery represents a practical implementation of this requirement despite a lack of information about how beneficiaries value the relevant ecosystem services. A formal ecosystem assessment could provide necessary information on the wider suite of ecosystem services that fishing might interact with and how beneficiaries value these services. Such information is likely to aid the future development of krill fishery management and help remove the current reliance on interim measures. Formal and comprehensive ecosystem assessment would require considerable investment but could substantially improve coordination between management bodies focused on different human activities at both the regional and global scale.
Acknowledgements
This paper is a contribution to the Natural Environment Research Council core-funded British Antarctic Survey Ecosystems programme. We are grateful to Sigve Nordum of Aker Biomarine for supplying some of the information presented in Table II.