Introduction
Chronic kidney disease (CKD) is now recognised as a major world-wide health problem (Davis et al., Reference Davis, Harmon, Himmelfarb, Hostetter, Powe, Smedberg, Szczech and Aronson2008). It is estimated that the prevalence of stage 3–5 CKD in the adult population of the United Kingdom is currently around 7% (Stevens et al., Reference Stevens, O'Donoghue, de Lusignan, Van Vlymen, Klebe, Middleton, Hague, New and Farmer2007; de Lusignan et al., Reference de Lusignan, Chan, Gallagher, van Vlymen, Thomas, Jain, Tahir, Nation, Moore, Reid, Harris and Hague2009), although only a very small minority may eventually require dialysis or a renal transplant. Diabetes mellitus has become the most common cause of CKD, not only within the developed world, but also increasingly within the emerging world, mainly due to the rise in the incidence of Type 2 diabetes (Atkins, Reference Atkins2005).
There has recently been a change in focus in managing CKD, from one of treating established kidney disease to one of earlier identification and prevention. As a result, there have been a number of important UK initiatives concerning the care of people with early CKD in recent years, including the publication of the National Service Framework for Renal Services Part Two (Department of Health, 2005) and the National Institute for Health and Clinical Excellence (NICE) guidance on CKD (NICE, 2008). In general terms, NICE helps those working in the health service and local authorities to deliver high-quality health care and also develops evidence-based guidelines on the most effective ways to diagnose, treat and prevent disease and ill-health. NICE also develops quality standards, gives recommendations for specific medications and has a wider remit to raise standards of healthcare around the world.
Collectively, these initiatives have had an enormous impact on the way in which people at risk of CKD are managed in both primary and secondary care. However, it is health-care professionals (HCPs) working in primary care, dealing with large numbers of people with CKD, who have the potential to prevent and delay the progression of the disease, in partnership with patients. Although efforts are being made nationally and locally to improve the management of patients with diabetic renal disease, a large number of patients still progress to established renal failure. Diabetes is the cause of CKD in 24% of new patients requiring dialysis in the United Kingdom. In some areas of high ethnic diversity, up to 35% of the dialysis population have diabetes (UK Renal Registry, 2010).
CKD has a huge impact on both quality of life and life expectancy for individuals with the condition and is an increasing public health concern. As over 2% of the total National Health Service (NHS) budget is spent on renal dialysis and transplantation, NICE (2008) recommended that initiatives that focus on early identification and prevention of CKD are required (NICE, 2008).
Background
Although several factors have been associated with an increased risk of developing diabetic kidney disease, no single factor has yet been shown to be predictive. People at risk are those with proteinuria, uncontrolled blood pressure (BP), poorly controlled blood sugar, those who smoke and those with a family history and/or specific ethnicity (Koppiker et al., Reference Koppiker, Feehally, Raymond, Abrams and Burden1998).
Many studies have shown that the course of diabetic kidney disease can be slowed by identifying those at risk and subsequently managing BP to target, improving glycaemic control and giving advice and support on lifestyle changes, such as exercise, weight loss and smoking cessation (DCCT Research Group, 1995; Gerstein, Reference Gerstein2002; Mancia, Reference Mancia2007; Bilous, Reference Bilous2008). However, there is evidence that risk factors for CKD and its progression are managed sub-optimally in primary care (New et al., Reference New, Middleton, Klebe, Farmer, de Lusignan, Stevens, O'Donoghue and Farmer2007).
One of the best ways to effectively manage diabetes is to empower patients with knowledge of their condition and likely outcomes. Most people with diabetes spend the majority of time managing their own condition, supported by HCPs for only a few hours each year. It is crucial that HCPs support people in managing their own diabetes, which in turn can improve patient-reported and clinical outcomes (Department of Health, 2001).
There is a question as to whether there is enough evidence to demonstrate that self-management is effective. A British Medical Association (BMA) (2007) report evaluated how far self-management could make a difference to long-term health outcomes. The BMA cites the Picker Institute review (Coulter and Ellins, Reference Coulter and Ellins2006), which reported that although a great deal of research had been undertaken into self-management, the majority of trials tended to measure only short-term outcomes, typically six months or less. The review concluded that even though there was currently little known about the effectiveness of self-management over the long term, self-management education can lead to short-term improvements in health behaviour.
A variety of reviews into self-management education programmes have concluded that, in general terms, small to moderate effects for selected chronic diseases can result. Effects include small but significant reductions in glycated haemoglobin levels (HbA1c) and improvements in systolic BP (Warsi et al., Reference Warsi, Wang, LaValley, Avorn, Solomon, Warsi, Wang, LaValley, Avorn and Solomon2004). In the short to medium term, diabetes self-management support can be effective (Glasgow et al., Reference Glasgow, Fisher, Skaff, Mullan and Toobert2007).
However, the evidence for effective self-management programmes for those with early CKD is variable (Curtin et al., Reference Curtin, Walters, Schatell, Pennell, Wise and Klicko2008). In Curtin's (Reference Curtin, Walters, Schatell, Pennell, Wise and Klicko2008) cross-sectional study in CKD, 174 patients completed self-reported measures of self-efficacy, physical and mental functioning and self-management. Findings showed that patients’ perceived self-efficacy was more consistently correlated with self-management behaviour than other characteristics such as demography.
In a review of all randomised controlled trials (RCTs) and quasi-RCTs studying the benefits of education for people with diabetes and CKD (Li et al., Reference Li, Wu, Wang, Huang, Yang, Dong and Liu2011), it was concluded that programmes can have positive effects on improving patients’ knowledge and also may result in behaviour changes that can improve health outcomes. Educational programmes also appear to have beneficial effects on improving patients’ self-efficacy and result in some belief changes for patients with diabetes and microalbuminuria (MA). However, only two studies that had small sample sizes and were of inadequate quality were included in this review. Li et al. (Reference Li, Wu, Wang, Huang, Yang, Dong and Liu2011) concluded therefore, that there was inadequate evidence to support the beneficial effects of education programmes for people with kidney disease caused by diabetes and suggested the need for further studies.
Methods
Design
The study aimed to investigate whether the parameters that can lead to deterioration of kidney function in diabetes can be better controlled through education provided in a self-management package. Ethical approval was granted by the local NHS Local Research Ethics Committee in December 2003. This was a mixed-method study that entailed two main separate stages:
1) Development of the self-management package.
2) Testing and evaluation of the self-management package.
The aims of the self-management package were:
• To inform people with diabetes of the risk factors for developing kidney damage.
• To provide key points for managing the ways in which kidney damage can be slowed down in people who are at risk.
• To give more detailed information on how they can self-manage their condition.
• To provide practical ways for increasing self-management.
Development of the self-management package has been reported (Thomas et al., Reference Thomas, Bryar and Makanjuola2008). Testing of the package was carried out in six GP Practices and one control Practice. The lead researcher recruited GP Practices to the study following educational sessions on CKD that were delivered in secondary care. At the start of the study, the lead researcher, a renal nurse, spent time in each Practice, observing diabetes clinics and offering clinical input when required. Clinical input included help with identifying and coding CKD, interpretation of quantification of proteinuria results (albumin–creatinine ratio (ACR)) and advice on BP control. Patient participants were invited to take part by either the lead researcher, or a practice nurse or GP in each participating Practice. A summary of the study design is shown in Table 1.
Table 1 Summary of study design

ACR = albumin–creatinine ratio; BMI = body mass index.
Data collection
Collected data items included systolic and diastolic BP; glycosylated haemoglobin (HbA1c); smoking status and body mass index. These are risk factors for diabetic kidney disease outlined in NICE guidelines (NICE, 2002). Data were collected manually from Practice systems (EMIS LV) and transferred to SPSS for Windows v12 for descriptive analysis. There were three data collections before the intervention (March 2005, October 2005 and March 2006); two data collections during the intervention (November 2006 and June 2007) and one data collection after the intervention (January 2008).
Participation
There were 496 participants (294 male and 202 female) in total at the start of the study, including the control group (n = 60). This equates to 23% of those with Type 1/Type 2 diabetes having MA. Of these, 76.3% were Caucasian, 2.5% African-Caribbean and 19.5% Asian. 6.4% had Type 1 diabetes and 93.6% had Type 2 diabetes. However, at the start of the intervention period, only 173 patients were eligible to take part in the self-management part of the study (see Table 2). Eventual participation in the self-management part of the study was achieved in 67% (116/173) of possible patients.
Table 2 Number of patients available for self-management intervention

MA = microalbuminuria.
Data collection in the control practice took place in December 2007, at the end of the data collection period. The control practice data were collected retrospectively from the date of extraction back to December 2004.
Analysis
A cluster design was adopted. Cluster design trials are those in which groups of patients rather than individuals are being investigated. The main consequence of adopting a cluster design is that the outcome for each patient can no longer be assumed to be independent of that for any other patient (Campbell and Grimshaw, Reference Campbell and Grimshaw1998) and patients within any one cluster are more likely to have similar outcomes. In this study, all the patients in one Practice were allocated to the same intervention, so each GP Practice formed a cluster.
The calculation required to adjust the sample size to take account of the clustering was adapted from Bland (Reference Bland2004) and the median intra-cluster correlation coefficient used was that reported in a systematic review of trials in primary care (Eldridge et al., Reference Eldridge, Ashby, Feder, Rudnicka and Ukoumunne2004). In total, 252 packs needed to be distributed for the study to be powered (42 patients per Practice needed to detect a difference with 90% power at 5% level of significance).
Results
Participation
A total of 92 participants who had MA at the start of the study did not have MA at the start of the intervention period. This may be due to two reasons. First that many participants had borderline MA and in addition, a small percentage of ACRs may have been false positives caused by infection. However, findings from a systematic review (Carter et al., Reference Carter, Tomson, Stevens and Lamb2006) concluded that it is unnecessary to screen asymptomatic patients with demonstrable proteinuria or albuminuria for urinary tract infection. What is more likely is that lack of progression of MA was related to timely prescription of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs; Araki et al., Reference Araki, Haneda, Koya, Kashiwagi, Uzu and Kikkawa2008). Rapid initiation of ACEIs/ARBs in people with MA can reap rewards especially if implemented alongside other measures such as strict BP and blood sugar control. It is possible that the participating Practices provided improved care to this cohort of people at risk of CKD following the initiation of the study by improving the screening rates for MA and by timely prescribing of appropriate medication to those at risk.
Despite excellent support from the participating Practices, it proved challenging to reach all suitable patients. All patients were asked whether they wished to participate either during routine appointments (annual diabetes review), ad hoc consultations or by letter. Of 173 patients, 134 were requested to take part by a practice nurse or GP, or sent a letter by the lead researcher, and of these 18 people refused to participate. Reasons for non-participation included being ‘not interested’, ‘too old for that sort of thing’ and literacy problems (including native English speakers). It proved difficult to contact the remainder, mostly younger people who were employed, especially if the participant was well and was not due at an imminent annual review appointment.
Participation in the study was achieved in 67% (116/173) of possible patients. In order for the study to have 90% power with 5% significance, it was necessary to distribute the self-management packs to 42 patients in each surgery, that is, 252 packs in all. As the study is underpowered, results should be viewed with caution, although the difficulties in achieving participation provide important messages about self-management programmes (see the section ‘Discussion’). It is also possible that some people took part but did not actually use the pack at all. An important question is how far the general public and those with long-term conditions believe that self-management or self-care is beneficial.
BP
At the end of the intervention period in January 2008, the patients who had received the self-management package had a mean systolic BP of 132.1 ± 14.2 mmHg versus 136.2 ± 16.4 mmHg in the control group (P = 0.15). Although not statistically significant, the group who received the self-management pack had a mean BP that is much nearer the NICE (2008) target of 130/80 mmHg for people with diabetes and CKD. Table 3 shows the mean systolic BP at different time points in the participating Practices. Systolic BP was reduced after the first four time periods but just fell short of statistical significance (P = 0.057). Table 4 shows the mean diastolic BP at different time points in different Practices. There was marginal significance in diastolic BP at time point 5 (P = 0.053).
Table 3 Mean systolic BP (mmHg) at March 2005 and January 2008
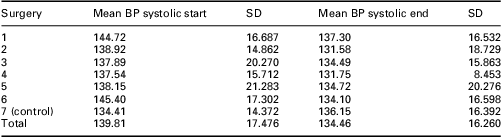
BP = blood pressure.
Table 4 Mean diastolic BP (mmHg) at March 2005 and January 2008
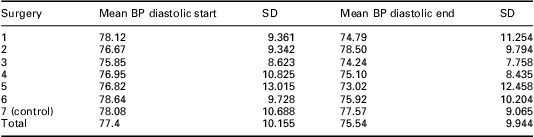
BP = blood pressure.
As the study was underpowered, it was not possible to identify significant differences between the intervention and control groups. At time points where there were marginal significant differences in systolic BP between the two groups, it is possible that, if the study had been powered, significant differences may have been identified.
Similar effects were not seen for control of blood sugar (HbA1c) and weight loss (body mass index). This may be because blood sugar and body weight are more difficult to control from an individual's perspective, and rely much more on behaviour change (diet, exercise) than change in concordance with medication.
Qualitative evaluation: the patient perspective
At the end of the distribution period, it was planned that a small number of people who had received the pack would help to evaluate the contents of the pack. The original study protocol stated that evaluation of the pack would be carried out with 15 patients using a short semi-structured questionnaire. The aim was to find out whether patients understood the content of the pack and whether they could make any recommendations for improvement.
Questionnaires were distributed by post and if not returned within three weeks people were sent a reminder. Only five replies were received despite the reminders and the data collected were not of much value except for some responses about the DVD:
…the DVD was fiddly and required too much effort – there was no compelling reason to watch it
the DVD reminded me what I needed to do.
Because of the small number of respondents, three face-to-face semi-structured interviews were undertaken with patients, to elicit further in-depth evaluation. In summary, all three looked at the pack either immediately after receiving it or within one week. Patients were asked which parts of the package were most useful:
I have always been a big reader, so I like the written information. If you see a film you can miss bits, so I would always prefer the piece of paper.
The bit about BP control, I took note of that. My BP is always high so that was very interesting.
Not that I can remember – it all seemed fine.
You could elaborate on the seriousness of it all. People don't realise how important it is.
Some additional questions based on the comments received from the questionnaire were asked, especially about the DVD as there was concern that some parts of the DVD might be distressing:
No, (I did not find the DVD distressing) not at all!
No I did not find it distressing at all, everything was perfect. I understood what it meant, and what that bit meant to say.
I don't think it was frightening at all….you should tell people the worst that can happen.
It was interesting to find out about any behaviour change that had resulted after viewing the pack:
I did not realise how important the monitoring of the diabetes and also the BP was, for kidney damage. But apart from taking the tablets then I'm not sure what I can do about that.
I am quite pleased with myself as I've given up smoking now.
Discussion
It is recognised that there are a number of stages between the giving of information (the intervention) and possible changes in outcome, such as reduced BP and HbA1c. The American Diabetes Association (Funnell et al., Reference Funnell, Brown, Childs, Haas, Hosey, Jensen, Maryniuk, Peyrot, Piette, Reader, Siminerio, Weinger and Weiss2008) has published national standards for diabetes self-management education. Standards 7–10 are concerned with patient aspects, most importantly individualised assessment, goal setting and effectiveness of the programme. Goal setting in diabetes education often relates to Social Cognitive Theory (Baranowski et al., Reference Baranowski, Perry and Parcel2002) and includes the process of identifying behaviours (self-control), greater confidence to perform behaviours (self-efficacy; Krichbaum et al., Reference Krichbaum, Aarestad and Buethe2003), positive reinforcement and other constructs presumed to maximise goal (behaviour) attainment (Sprague et al., Reference Sprague, Shultz and Branen2006).
The Department of Health published findings of a longitudinal study on public attitudes towards self-care in 2008 (Department of Health, 2008). The study investigated the perceptions of the general public and people with long-term conditions about their attitudes towards self-care and found that 89% of those asked from the general public, felt that people should take responsibility for their own well-being. However, for those with a long-term condition, the results found that 75% of those questioned (n = 1975), said they played an active role in treating their condition ‘all or most of the time’. An important issue that was raised was how many with a long-term health condition (22%) were unable to perceive an advantage in taking a greater role in care of their health and condition and a further 15% did not know whether or not there were any advantages. The potential is clearly there to increase awareness among the advantages of self-care, and a recommendation from this study is that people with diabetes and CKD should have a full explanation of the advantages of self-care, especially potential slowing of kidney disease progression.
The National Diabetes Audit (NDA) of 2005–2006 (The Information Centre National Clinical Audit Support Programme, 2006) showed that 26.8% of people with diabetes achieved the NICE (2002) BP target of <135/75 mmHg. By comparison, 58% of participants at the start of this study, had achieved the NICE target of 135/75 mmHg. It is difficult to compare these data directly, as all of the participants in this study had MA and the percentage of those with MA in the national audit sample was not stated. However, it appears that overall BP control in the participating practices at the start of the study was better than in the national sample. The later NDA Audit of 2009–2010 showed that people with Type 2 diabetes are less likely to achieve their BP target than people with Type 1 diabetes. In addition, many people with kidney disease who achieve the 140/80 mmHg target do not achieve the recommended NICE (2008) target of 130/80 mmHg target.
It is therefore likely that changes to BP in the intervention practices have occurred as a result of this study's interventions rather than external influences. What cannot be explained is the exact reason for these resulting reductions in mean BP. It is possible that effects came about because of direct patient behaviours, such as individuals understanding of the risks of high BP and being more concordant with prescribed anti-hypertensive medications. It is possible that there was an effect resulting from the researcher's visits to the intervention practices, and awareness of CKD prompted more diligent monitoring, recall and prescribing of anti-hypertensive medication.
It is possible that the practitioners’ confidence in managing BP to target improved as the study progressed, and this in turn improved BP control. This increased confidence may have resulted as a consequence of the researcher's visits to the practice or as a result of Quality and Outcomes Framework (QOF) incentivisation (Carey et al., Reference Carey, Nightingale, DeWilde, Harris, Whincup and Cook2009). It is difficult to extrapolate whether practitioners’ titrated BP medication to maximum levels to achieve QOF targets, whether they invested more time in explaining the benefits of BP tablets to slow down kidney disease progression, or a mixture of the two. A refinement to the intervention could be the inclusion of a ‘Confidence in Managing CKD’ questionnaire (Tahir et al., Reference Tahir, Gallagher, Thomas, Harris and de Lusignan2011) undertaken before and after the intervention, which would identify in part whether changes in outcome were directly due to practitioner interventions. It would also have been helpful if pre- and post-testing of patient knowledge or self-efficacy had been carried out to identify engagement with, and the impact of, the package.
The difficulties in isolating the ‘active ingredient’ of a self-management intervention are well known, and perhaps interventions such as these could be defined as complex. The Medical Research Council (MRC, 2000) described a complex intervention as follows:
Complex interventions in health care, whether therapeutic or preventative, comprise a number of separate elements which seem essential to the proper functioning of the intervention, although the active ingredient that is effective is difficult to specify (MRC, 2000)
It has been recommended that complex interventions should be carefully planned and designed and to help researchers, the MRC in 2000 published a five-phase framework for developing and evaluating complex interventions. The five phases are pre-clinical, modelling, exploratory, definitive RCT and long-term implementation. The updated MRC Framework guidance recommends that researchers ask specific questions of the intervention during each phase (MRC, 2008). The first three phases of the MRC Framework were undertaken in this study: pre-clinical (literature review) phase, modelling (use of theory) phase and exploratory trial phase, yet further analysis of these stages is warranted.
It is recommended that Phase IV includes an RCT, so a definitive RCT in the future is being considered, although it is not that realistic to undertake an RCT (at patient level) because of practitioner effects in one GP practice. If the intervention was randomised at patient level, then practitioners might find it difficult to provide the intervention in one patient followed by unchanged usual practice in the next. In addition, individual practices might have differing populations, differing QOF achievement scores or differing approaches to self-management. A cluster-randomised trial (CRT, at practice level) would be the method of choice. However, it is recognised that it is not possible to fully account for all effects such as practitioner confidence in managing diabetes/CKD and practitioner confidence in facilitating a self-management approach, although if these measures are incorporated into the design of the Phase IV study, then the effects of these variables might to some extent be reduced.
The findings show that it is possible to achieve the NICE (2008) BP target of 130/80 mmHg for people with CKD and diabetes. This is in contrast with some other studies that found BP in people with diabetes difficult to control. One review (McLean et al., Reference McLean, Simpson, McAlister and Tsuyuki2006) found that fewer than one in eight people (n = 47 964) with diabetes and hypertension have adequately controlled BP (defined by BP 130/85 mmHg), and recommended that there was an urgent need for multidisciplinary, community-based approaches to manage this high-risk cohort. A primary care study in Nottingham (Bebb et al., Reference Bebb, Coupland, Stewart, Kendrick, Madeley, Sturrock and Burden2007) found that only 46% of participants had well-controlled BP (defined by BP <145/85 mmHg). Recent figures from the NDA in England (2011), showed 63.3% reaching their BP target in Type 1 diabetes and 49.5% in Type 2 diabetes.
One of the main aims for primary care management of diabetes is to minimise the risk of cardiovascular complications. The main finding from this study is that self-management techniques such as understanding of, and subsequent concordance with, prescribed medication may provide the opportunity for an individual to control their own BP. This assertion is supported by the downward trend in both mean systolic and diastolic BP recordings in the participating practices during time periods 1–4, before the intervention being rolled out. The importance of maintaining BP to target is that it can slow the rate of CKD progression and reduce cardiovascular risk (Bilous, Reference Bilous2008).
In terms of cardiovascular risk reduction the reduced BP in the intervention group has important implications. Numerous studies have shown that BP control is an important modifiable risk factor for cardiovascular events (Mourad and Le Jeune, Reference Mourad and Le Jeune2008). BP control with ACE inhibitors in the MICRO-HOPE study showed significant reductions in the risk of cardiovascular events and appropriate prescribing of ARBs produced renal protection in patients with diabetes and high BP (Gerstein, Reference Gerstein2002). An intensive intervention that included BP control was undertaken in the Steno-2 study. Results from this study showed a sustained reduction in the risk of cardiovascular complications and death (Gaede et al., Reference Gaede, Vedel, Larsen, Jensen, Parving and Pedersen2003).
Although the BP endpoints from this current research study did not reach the NICE (2008) recommendations of <130/80 mmHg, a differential of >3 mmHg compared with the control group might have significant effects on cardiovascular risk reduction.
Limitations of the study
Researcher influence
It is also possible that active involvement from a renal nurse in identifying abnormal ACR results and subsequent initiation of medicines that modify the renin–angiotensin pathway, may also have an effect on BP control.
Practitioner influence
There may have been differences between Practices, as some practitioners may have used a facilitative approach when explaining the aims of the pack (emphasising what the patient can do to help themselves), whereas others may have focussed on what should be done or not done, such as stopping smoking. Although further evidence is needed to clarify how far the performance of diabetes educators can influence outcomes (Loveman et al., Reference Loveman, Frampton and Clegg2008), further guidance for practitioners would have been beneficial. In addition, a guide for practitioners that explained how to answer patients’ questions that arose as a result of the intervention may also have contributed to removing practitioner bias.
The intervention developed for this study did not explicitly include the assessment and goal-setting stages, although these stages might have been included in generic one-to-one discussions with practice nurses during annual review clinics or regular check-ups. It is recognised that the omission of these stages is a limitation of this current study.
Effect of national policy
The care of people with CKD in primary care has changed dramatically over the past five years. This raises the question as to whether the differences in clinical parameters found in the intervention group in this study might have happened as a result of the national initiatives. The amendment to the method to include a control group did aim to eradicate the effect of the policy changes. Although there was a downward trend in mean systolic and diastolic BPs in the intervention group, which was not seen in the control group, it is possible that national policy changes did affect the management of patients in the participating practices observed by the falling mean BP recordings that occurred before the intervention was rolled out.
Even bearing this in mind, it is likely that the effect on BP seen in the study is an effect that has been achieved by the education pack rather than the effect of the researcher visiting the intervention practices as it was an effect seen to occur/continue after the distribution of the packs and withdrawal of the researcher from the practices. It was not possible at the outset of the study to predict the huge changes in national policy and publicity surrounding the management of kidney disease in primary care during the study period 2004–2009. If this had been known at the start an alternative method could have employed, such as a CRT. Practices could be recruited as before, but randomised (at practice level) to the intervention or to usual practice.
Relevance to clinical practice (recommendations)
This study gives rise to a number of important recommendations. First, for primary care practitioners to ensure they are confident in identifying and managing people with diabetes who are at risk of CKD. Especially important is the prescription of ACE/ARBs once MA is detected and also that practitioners impart strategies for self-management to patients.
From a patient perspective, it is crucial that people at risk of CKD understand their individual BP targets and the type of medication they are prescribed to control BP. An important message is that ACE/ARBs have a dual role in managing MA and also BP. Also that BP medication often has side effects (troublesome cough or swollen ankles) and that reporting of these side effects is helpful. Patients should also be advised about other strategies that can avert renal disease progression, such as smoking cessation.
The importance and possible benefits of self-care were highlighted by some patients during the evaluation stage as not receiving enough emphasis, so it is recommended that self-management interventions for patients should include clear messages about how self-management can be beneficial and also contain recommendations that patients prepare questions for their GP or practice nurse before a consultation visit.
To promote practice nurse and GP engagement with self-management techniques, education and training in how to promote self-management skills for patients is recommended. Innovative teams are now developing self-management training sessions for health-care professionals that are delivered by patients (Loud et al., Reference Loud, Jain, Thomas and Gallagher2011).
Conclusion
Self-management techniques such as understanding of, and subsequent concordance with, prescribed medication may provide the opportunity for an individual to control their own BP. The importance of maintaining BP to target is that it can slow the rate of CKD progression and reduce cardiovascular risk (Bilous, Reference Bilous2008).
The methods used in this study could be replicated for other long-term conditions, although the simple action of self-management pack development and distribution needs to be accompanied by a commitment to a self-care ideology. A culture change from a passive to an active self-care philosophy requires the support of lead clinicians responsible for managing long-term conditions.
Acknowledgements
To Dr David Makanjuola for his suggestions for study design and data analysis. To Fiona Warburton for assistance with statistical analysis. Authorship: Study Design (N.T. and R.B.); Data Collection and Analysis (N.T.); and Manuscript Preparation (N.T. and R.B.). Sources of support: British Renal Society/Kidney Research UK Fellowship; SW Thames Kidney Fund; St Helier Association of Kidney Patients (SHAK); Insulin-Dependent Diabetes Trust; and Hospital Savings Association (HSA).








