INTRODUCTION
The first reports of human infection with influenza A(H1N1) 2009 (2009 H1N1) occurred in early April 2009 in southern California and Mexico. By late April, the virus had spread to other regions of the world [1]. On 11 June 2009, the World Health Organization declared the first Phase 6 global influenza pandemic of the century [2].
In Korea, the first case of 2009 H1N1 was documented on 2 May 2009. Subsequently, confirmed cases in Korea were reported mainly in overseas travellers or in those who had come into direct contact with infected people. By October 2009, the incidence of 2009 H1N1 had significantly increased and by 17 April 2010, about 750 000 Korean patients were confirmed as having 2009 H1N1 infection and 252 had died [3]. The clinical features and disease course of critically ill patients have been described for several countries in the northern and southern hemispheres that were affected by 2009 H1N1 [Reference Dominguez-Cherit4–Reference Rello7]. The characteristics of 2009 H1N1 compared with seasonal influenza were younger age and higher rates of obesity and pregnancy in critically ill patients. However, little information was available concerning Korean patients who became critically ill after infection with 2009 H1N1. Here, the characteristics, clinical features, treatments and outcomes of adult Korean patients who became critically ill after being infected with 2009 H1N1 influenza are described.
METHODS
We retrospectively reviewed the medical records of all adult patients with confirmed 2009 H1N1-related critical illness who were treated at the 28 participating hospitals in South Korea between 1 September 2009 and 28 February 2010. This study was approved by the local institutional review board. Informed consent was not required because this was not an interventional study.
Eligible patients were aged ⩾15 years, were critically ill and had been admitted to one of the 28 study hospitals with confirmed 2009 H1N1 infection. Infection with 2009 H1N1 was confirmed by testing a sample acquired via nasopharyngeal swab or bronchoalveolar lavage (BAL), and obtaining a positive result in a probe-based reverse transcriptase–polymerase chain reaction (RT–PCR) for the 2009 H1N1 virus. Critically ill patients were defined as those who (i) were admitted to the intensive-care unit (ICU) or required mechanical ventilation (i.e. invasive or non-invasive); (ii) had a ratio of partial pressure of oxygen in arterial blood (PaO2) to inspired fraction of oxygen (FiO2) <300 mmHg; or (iii) required intravenous infusion of an inotropic or vasopressor medication. First, the patients were identified by searching the databases of the microbiology laboratory. Patients were then included on the basis of critical illness criteria. Eligibility criteria were confirmed by site investigators at each centre and data were recorded by trained research nurses or site investigators at each centre.
We reviewed the medical records of the patients and recorded the following data: date of admission to hospital and the ICU, age, sex, weight and height [for calculation of body mass index (BMI)], date of first symptoms, laboratory data, radiographic findings and comorbidities. Severity of illness was assessed using the Acute Physiology and Chronic Health Evaluation (APACHE) II and the Sequential Organ Failure Assessment (SOFA) scores on the day of admission. Nosocomial influenza acquisition was defined as the onset of influenza-like symptoms at least 72 h after hospital admission. Nosocomial bacterial pneumonia was defined as microbiological evidence for bacterial infection that was acquired more than 48 h after hospital admission, with clinical features compatible with bacterial pneumonia and with one or more of the following signs and symptoms: production of purulent sputum or a worsening in sputum character; fever or hypothermia (oral temperature ⩾38 °C or ⩽35·5 °C); systolic blood pressure (BP) <90 mmHg; and total leukocyte count >10 000/μl, leukopenia (total leukocyte count <4500/μl) or >15% immature neutrophils regardless of total leukocyte count. In addition, a patient was required to have a chest radiograph consistent with the diagnosis of pneumonia (new or progressive infiltrates, consolidation, with or without pleural effusion). The primary outcome measures consisted of mortality at 30 days and 90 days after onset of symptoms. Secondary outcomes included frequency and duration of mechanical ventilation and duration of ICU and hospital stay.
Statistical analysis
Categorical variables were presented as numbers and percentages and were compared using the χ2 test or Fisher's exact test. Continuous variables were expressed as means [standard deviation (s.d.)] or medians [interquartile range (IQR)] and were compared using Student's t test or Mann–Whitney test. To evaluate 90-day mortality, logistic regression analysis was performed. Variables with a P value <0·15 in univariate analyses were candidates for the multivariate logistic model. A backward elimination process was used to develop the final multivariate model, and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. A P value <0·05 was considered statistically significant. All analyses were performed using SPSS for Windows release 18.0 (SPSS Inc., USA).
RESULTS
Characteristics of study hospitals and patients
A total of 245 critically ill patients infected with H1N1 were admitted to the ICUs of 28 participating hospitals [ICU beds (median 20, IQR 15–24); total hospital beds (median 965, IQR 908–1200)] between 1 September 2009 and 28 February 2010. Twenty-two hospitals (78·6%) were university or university-affiliated hospitals.
The mean (±s.d.) age of the 245 critically ill patients was 55·3±18·4 years (range 15–93 years) and 68·6% of the patients were aged >50 years. Table 1 provides additional characteristics of the 245 patients. Upon initial presentation, the mean (±s.d.) APACHE II score was 19·1±8·4. A total of 205 (83·7%) patients had one or more comorbidities. The most common comorbidities were hypertension (35·9%), smoking (28·6%), malignancy (27·8%), chronic lung disease (25·8%) and diabetes (24·5%). The BMI data were available for 184 patients. The mean (±s.d.) BMI was 22·9±4·3 kg/m2 and only nine (3·7%) patients were obese (BMI >30 kg/m2). Three of the patients were pregnant. Of these, two were in the third trimester and one in the first trimester. Thirty-five (14·3%) patients had been infected via nosocomial transmission, none of whom were healthcare workers. Nosocomial acquisition predominantly affected older subjects (mean age±s.d.=60·4±13·4 years) who had several comorbidities [malignancy (n=22, 62·8%), cerebrovascular disease (n=9, 25·7%), chronic lung disease (n=8, 22·9%), liver cirrhosis (n=5, 14·3%)].
Table 1. Baseline characteristics of critically ill patients with confirmed pandemic influenza A(H1N1) 2009
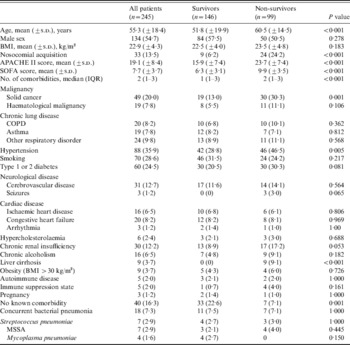
APACHE II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; MSSA, methicillin-susceptible Staphylococcus aureus; SOFA, Sequential Organ Failure Assessment.
Values given are n (%) unless stated otherwise.
Coexistent bacterial pneumonia on admission was diagnosed in 18 (7·3%) patients. The most common presenting symptoms were respiratory symptoms such as shortness of breath (74·3%), cough (60·4%), sputum production (52·7%) and fever (65·3%). Leucocytosis was present in 48·2% of the patients and lymphopenia in 82·4%. Based on chest radiography at presentation, 164 (66·9%) patients had bilateral infiltrates (Table 2). Patients who died were more likely to have higher APACHE II and SOFA scores (Table 1) and lower mean arterial pressure, PaO2/FiO2 ratio, platelet count and lymphocyte count at admission (Table 2).
Table 2. Clinical features upon intensive care unit admission
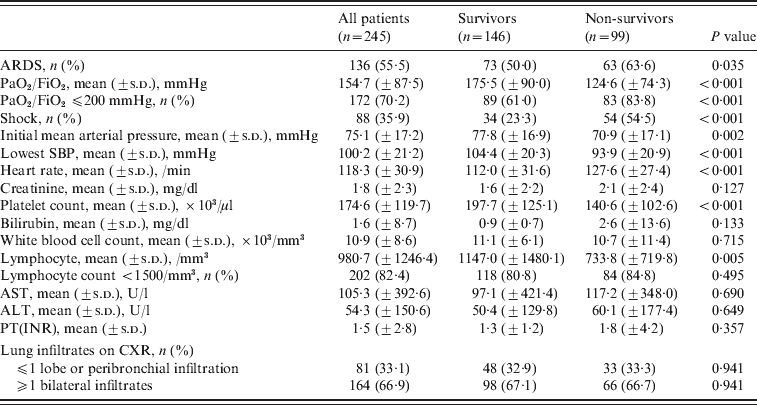
ALT, Alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; CXR, chest X ray; ICU, intensive care unit; PT(INR), prothrombin time (international normalized ratio); SBP, systolic blood pressure.
Course of illness and treatments received
The median time from symptom onset to presentation was 3 days (IQR 1–4 days) and the median time from presentation to ICU admission was 1 day (IQR 0–2 days) (Table 3). All patients received antiviral treatment. Of these, 112 (45·7%) patients received high-dose oseltamivir (300 mg/day). Twenty (8·2%) and 39 (15·9%) patients received double (i.e. oseltamivir and amantadine or ribavirin) or triple (i.e. oseltamivir, amantadine, ribavirin) combination regimens, respectively. Only 42·9% received antiviral therapy within 48 h of symptom onset. The duration from symptom onset to initiation of antiviral agents was not significantly different between survivors and non-survivors [3 days (IQR 1–5 days) and 3·5 days (IQR 2–6 days), respectively, P=0·078]. There were also no significant differences in antiviral regimen or dose between survivors and non-survivors. Fourteen of the 17 patients who received rescue therapy died. Other medical treatments included antibiotics (243 patients, 99·2%), corticosteroids (107 patients, 43·7%) and diuretics (153 patients, 62·4%).
Table 3. Clinical course and treatment of critically ill patients with confirmed pandemic influenza A(H1N1) 2009

ECMO, Extracorporeal membrane oxygenation; ICU, intensive-care unit; IQR, interquartile range; MV, mechanical ventilation; NIV, non-invasive ventilation; NO, nitric oxide; PEEP, positive end-expiratory pressure.
Values given are n (%) unless stated otherwise.
* Oseltamivir and amantadine or ribavirin.
† Oseltamivir and amantadine and ribavirin.
A total of 164 (66·9%) patients underwent mechanical ventilation for a median of 7 days (IQR 3–16·5 days). Initially, 151 (61·6%) of these procedures were invasive and 13 (5·3%) were non-invasive. Ultimately, 11/13 patients who received non-invasive ventilation required invasive ventilation. On the first day of ICU admission, the mean (±s.d.) PaO2/FiO2 ratio was 154·7±87·5 mmHg, the mean (±s.d.) FiO2 value was 73·2±22·2% and the mean (±s.d.) positive end-expiratory pressure (PEEP) was 9·1±4·0 cmH2O. Rescue therapies for oxygenation failure required neuromuscular blockade in 106 (43·3%) patients, prone positioning ventilation in 21 (8·6%) patients, inhaled nitric oxide in 13 (5·3%) patients and extracorporeal membrane oxygenation in 12 (4·9%) patients. Fifty-five (22·4%) patients became dependent on haemodialysis during their stay in the ICU. The median length of ICU stay was 7 days (IQR 3–14 days) for all patients, 6 days (IQR 3–13 days) for survivors and 8 days (IQR 4–20 days) for non-survivors (P=0·015). The median duration of ventilation was 7 days (IQR 3–16·5 days) for all patients, 8 days (IQR 3–14 days) for survivors and 7 days (IQR 3–17 days) for non-survivors (P=0·406). Patients who died had more severe hypoxaemia, multisystem organ failure, a requirement for frequent and prolonged mechanical ventilation or haemodialysis, and delayed ICU admission (Table 3).
Barotrauma occurred in 14 (5·7%) patients during mechanical ventilation. Nosocomial bacterial pneumonia developed in 78 (31·8%) patients during the course of treatment. Eleven (4·5%) patients became infected with fungus. The frequency of nosocomial pneumonia caused by carbapenem-resistant Acinetobacter baumannii (CRAB) and Pseudomonas aeruginosa was particularly high in non-survivors (Table 4).
Table 4. Complications during treatment

CRAB, Carbapenem-resistant Acinetobacter baumannii; MRSA, methicillin-resistant Staphylococcus aureus.
Values given are n (%).
Outcomes
Ninety-nine (40·4%) of the 245 patients died within 90 days of symptom onset, 131 recovered and were discharged from hospital within 60 days and 15 remained in hospital for longer than 60 days. Fifty-four (54·6%) of those 99 patients died within the first 14 days of symptom onset and 80 (80·8%) died within 30 days of symptom onset. Seventy-six (76·8%) of the 99 patients who died were aged >50 years. Twenty-five (71·4%) of the 35 patients who were infected by nosocomial transmission died. Of the three pregnant women, one experienced a miscarriage and one died.
Risk factor analysis
Univariate and multivariate analyses of risk factors associated with 90-day mortality are given in Table 5. Multivariate logistic regression analysis revealed that old age, SOFA score, clinician's decision to prescribe corticosteroids and nosocomial bacterial pneumonia caused by CRAB were independent risk factors for 90-day mortality. In addition, in order to explore the potential for a mortality time bias (patients need to survive long enough to receive a treatment, therefore, such treatment-related variables may be more likely to show an association with survival), we restricted our analysis of 90-day mortality to patients (n=230) who survived longer than the first 3 days. The associations and significance were attenuated only mildly.
Table 5. Risk factors associated with 90-day mortality

APACHE II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; CI, confidence interval; CRAB carbapenem-resistant Acinetobacter baumannii; ICU, intensive-care unit; OR, odds ratio; RRT, renal replacement therapy; SOFA, Sequential Organ Failure Assessment.
DISCUSSION
In South Korea, critically ill adult patients infected with 2009 H1N1 were most commonly in older age groups. Hypertension, malignancy and chronic lung disease were common comorbidities, but in contrast with other studies [Reference Dominguez-Cherit4, Reference Kumar5, Reference Rello7, Reference Estenssoro8], obesity was rare and only three patients were pregnant. The frequency of nosocomial acquisition was high and was associated with high mortality. Respiratory failure progressed rapidly in some patients, necessitating prolonged mechanical ventilation or frequent use of rescue therapies. Although some patients were given high-dose oseltamivir or triple combination antiviral therapy, there was no significant difference in the survival rates with these treatment regimens. Multivariate logistic regression analysis revealed that the clinician's decision to prescribe corticosteroids, older age, SOFA score and nosocomial bacterial pneumonia caused by CRAB were independent risk factors for 90-day mortality.
In this study, in contrast with data from other countries [Reference Dominguez-Cherit4, Reference Kumar5, Reference Rello7, Reference Davies9, Reference Hancock10], 68·6% of the infected patients who developed severe disease were aged >50 years, which is higher than the percentages reported in Canada (25%) [Reference Kumar5], France (48%) [Reference Fuhrman11] and Spain (28–31%) [Reference Rello7, Reference Viasus12]. In Korea, as in other countries, 2009 H1N1 affected mostly younger people, but critical illness occurred mainly in older patients [Reference Kim13, Reference Shin14]. Thus, we believe that not only is this study not biased but also that it is representative of the Korean adult acutely critically ill patients with 2009 H1N1. This study showed that the age-related mortality rate had a J-shaped curve, as did the studies of Kim et al. [Reference Kim15] and Echevarria-Zuno et al. [Reference Echevarria-Zuno16]. However, Sundar et al. reported that in the USA, 87% of deaths occurred in those aged <65 years [Reference Sundar17]. Our study supports the perspective that great heterogeneity among regions in terms of the incidence and mortality of infection is a characteristic of pandemics [Reference Miller18].
Compared with a previous study, the frequency of nosocomial acquisition observed in our population was high. Most cases of nosocomial acquisition occurred in older subjects with chronic comorbidities. Several reports have shown nosocomial influenza acquisition in patients with haematological malignancy [Reference Gooskens19–Reference Nicoll21]. In our study, 62·8% of patients with nosocomial acquisition had haematological or other malignancies. The presence of an immunocompromised state such as malignancy might be a risk factor for nosocomial influenza acquisition. Although there are insufficient data about nosocomial influenza acquisition, it is necessary to establish guidelines for prevention of nosocomial transmission of 2009 H1N1 and reinforce surveillance because of the high mortality resulting from an immunocompromised state, delayed treatment and transmission of resistant organisms [Reference Gooskens19, Reference Poalillo, Geiling and Jimenez22].
The 2009 H1N1 virus is associated with many of the same comorbidities as seasonal influenza, except for obesity and pregnancy [Reference Dominguez-Cherit4–Reference Rello7, Reference Vaillant23]. Obesity and pregnancy in patients were rare in this study. Louie et al. [Reference Louie24] reported obesity as a novel risk factor for 2009 H1N1 and Kim et al. [Reference Kim13] published a report of mortality in 115 Korean cases with a frequency of BMI >25 for 23% and no pregnancy. However, two multicentre studies from Canada and Mexico also showed that obesity was not a risk factor for 2009 H1N1 [Reference Dominguez-Cherit4, Reference Kumar5]. Additionally, Yu et al. showed that pregnancy was rare in critically ill patients in China [Reference Yu25]. We suggest that these risk factors might be subject to regional or racial differences, and that further study of this issue is needed. In this study, more than 80% of our patients had a comorbidity such as malignancy (solid cancer and/or haematological malignancy) or chronic lung disease. The high incidence of malignancy in our patients may reflect the fact that this study was mainly conducted at tertiary referral hospitals. Patients with malignant diseases are immunocompromised, and death from influenza-related infections is more common in cancer patients [Reference Cooksley26].
Only 42·9% of our patients received antiviral agents within 48 h of symptom onset. There was no significant difference between survivors and non-survivors in the period of time from symptom onset to initiation of treatment with antiviral agents. Some studies showed that severe 2009 H1N1 infection that required admission to an ICU was associated with a longer period of time between symptom onset and initiation of antiviral therapy [Reference Zarychanski27–Reference Yu29], but a recent meta-analysis showed that early antiviral therapy did not decrease lower respiratory tract complications [Reference Jefferson30]. Thus, it is possible that the initial antiviral treatment seldom, if ever, influences the fate of rapidly fatal outcomes of 2009 H1N1. The hypothesis that there is a physiological ‘point of no return’ prior to antibiotic therapy has been confirmed in sepsis of critically ill patients [Reference Garnacho-Montero31]. Some reports have found that high-dose oseltamivir (300 mg/day) may be more effective for H5N1 (avian influenza) in patients with severe pulmonary disease [Reference Abdel-Ghafar32]. Nguyen et al. [Reference Nguyen33] reported that the components of the triple combination treatment (i.e. oseltamivir, amantadine, ribavirin) act synergistically against influenza A. At present, there are insufficient clinical data on the efficacy of high-dose oseltamivir or triple combination regimens in the treatment of 2009 H1N1. Some of our 2009 H1N1 patients received high-dose oseltamivir or triple combination therapy, but there was no significant difference between survivors and non-survivors in the use of antiviral regimens. In Taiwan and Argentina, some patients received oseltamivir at a dose of 150 mg b.i.d., but the dosage of oseltamivir was not significantly associated with death [Reference Estenssoro8, Reference Chien28]. Although 17 patients in our study received peramivir as rescue therapy, only three survived. Thus, critically ill patients with 2009 H1N1 infection have a grave outcome despite rescue therapy with peramivir. The efficacy of peramivir as an early treatment in such patients, rather than as rescue therapy, should be further evaluated. Also lacking are clear data on the potential efficacy of corticosteroids in the treatment of severe acute respiratory distress syndrome (ARDS) resulting from 2009 H1N1 infection [Reference Martin-Loeches34]. Our multivariate logistic regression analysis revealed that the clinician's decision to prescribe corticosteroids was a potent independent risk factor for 90-day mortality. The use of dexamethasone did not reduce mortality in a murine model of ARDS infected with H5N1 [Reference Xu35], and a study of H5N1 influenza cases revealed a higher mortality rate in those treated with steroids than in the non-steroid-treated group [Reference Abdel-Ghafar32]. Lee et al. [Reference Lee36] reported that major comorbidities and systemic use of corticosteroids were associated with slower influenza clearance. A small number of studies have reported a rate of nosocomial bacterial pneumonia in patients with 2009 H1N1 of around 25% [Reference Rello7, Reference Estenssoro8]. In our study, the rate of nosocomial bacterial pneumonia was 32%. Use of corticosteroids could cause immunosuppression and secondary infection [Reference Sprung37–Reference Sprung39]. Although corticosteroid dose, timing of corticosteroid commencement and duration of corticosteroid treatment were not standardized between centres, these studies suggest that caution is necessary when administering corticosteroids to patients with influenza. Future studies are needed to assess the effects of different antiviral regimens and corticosteroids on 2009 H1N1-related critical illness.
In our study population, the 90-day mortality rate for critical illness relating to 2009 H1N1 was 40·4%, which was higher than in previous reports [Reference Dominguez-Cherit4–Reference Louie6, Reference Estenssoro8, Reference Davies9]. A couple of explanations may be advanced. First, some studies reported that the case-fatality rate was high in older patients (age ⩾50 years) [Reference Louie6]. Most of the patients in our study population were aged >50 years and 76·8% of the patients who died were aged >50 years. The high incidence of malignancy in our patients may also have contributed to the high mortality. Second, nosocomial bacterial pneumonia and fungal infection could have contributed to the mortality in this study.
This study has some limitations. As previously mentioned, the retrospective design may have resulted in selection bias. Furthermore, in some cases, we were unable to collect relevant patient data such as history of influenza vaccination and transmission route of nosocomial influenza infection, and the dose, timing and duration of corticosteroid treatment were not standardized among centres. In addition, the design of this study meant that causality could not be assessed and despite use of a multivariate regression model, confounders might still exist. This study was conducted mainly at tertiary referral hospitals and only included patients who were aged ⩾15 years – paediatric ICUs were not included in this study. Thus, it is possible that we underestimated the number of young and healthy patients infected with 2009 H1N1 influenza. All 28 hospitals that are members of the Korean Study Group for Respiratory Failure participated in this study voluntarily. The 28 hospitals were evenly distributed across the country. All of them were referral hospitals (22 of them were university or university-affiliated hospitals) so that most severely ill patients diagnosed in primary clinics might have been transferred to these hospitals. It could be possible that this group of patients received more specialized intensive care in these hospitals. However, when the national data for Korea were reviewed, we had included 99/252 (39·3%) patients in Korea who died with a diagnosis of H1N1 pneumonia [3].
CONCLUSION
In our Korean population, older patients with chronic comorbidities (especially hypertension, malignancy or chronic lung disease) were more likely to become critically ill with 2009 H1N1 influenza, but obesity and pregnancy were rare in infected patients. The frequency of nosocomial acquisition was high and associated with a high rate of mortality. Many of the study subjects received high-dose oseltamivir or a triple combination antiviral regimen after hospital admission. Nonetheless, the death rate was 40·4% in patients who became critically ill as a result of infection with 2009 H1N1.
ACKNOWLEDGEMENTS
We thank E. Cho, N. Lee (clinical research nurses), C. Lim, J. Huh, S. Yun, S. Choi (University of Ulsan College of Medicine), S. Koh (Yonsei University College of Medicine) and J. Marshall (St Michael's Hospital) for their assistance in the statistical review and the preparation of the manuscript. We also acknowledge the informative and insightful review by an anonymous reviewer.
APPENDIX. Korean Society of Critical Care Medicine H1N1 Collaborative
K. Jeon, Sungkyunkwan University School of Medicine; W. Choi, Keimyung University School of Medicine; J. Ahn, University of Ulsan College of Medicine; Y. Lee, Ajou University School of Medicine; H. Lee, Chonbuk National University Medical School; J. Kim, Chung Ang University College of Medicine; J. Cho, Inha University College of Medicine; H. Choi, Dongguk University Gyeongju Hospital; Y. Park, Hallym University School of Medicine; H. Kim, Gyongsang National University School of Medincine; Y. Kim, Chungnam National University Hospital; C. Lim, CHA University College of medicine; Y. Kim, Pusan National University College of Medicine; H. Kim, Korea Cancer Center Hospital; Y. Ryu, Ewha Womans University School of Medicine; M. Han, Eulji University School of Medicine; Y. Ko, School of Medicine Kyung Hee University; G. Chon, Konkuk University School of Medicine; K. Lee, College of Medicine Yeungnam University.
DECLARATION OF INTEREST
None.









