CVD, including CHD, stroke and peripheral vascular diseases, are clinical features of advanced atherosclerosis and are often associated with ageing( Reference Schwenke 1 ). CVD account for 70–80 % of deaths among men and women over 65 years of age( Reference Geelen, Lorga Filho and Primo 2 ).
The ageing process is accompanied by an increase in modifications to lipoproteins( Reference Reaven, Napoli and Merat 3 ) that result in a greater susceptibility of LDL and HDL to lipid peroxidation( Reference Khalil, Jay-Gerin and Fulop 4 , Reference Khalil, Wagner and Lacombe 5 ) and a reduction in the anti-atherogenic activity of HDL( Reference Gowri, Van der Westhuyzen and Bridges 6 , Reference Zago, Sanguinetti and Brites 7 ). A number of epidemiological and interventional studies have shown that there is an inverse relationship between plasma HDL levels and CVD( Reference deGoma, deGoma and Rader 8 ). This beneficial effect has been attributed principally to the capacity of HDL to promote cholesterol efflux from peripheral cells through reverse cholesterol transport and to protect LDL against oxidation and against the pro-inflammatory effect of oxidised LDL (oxLDL)( Reference Camont, Chapman and Kontush 9 ). HDL inhibit the expression of adhesion molecules on endothelial cells, contributing to the reduction in the recruitment of blood monocytes into artery walls( Reference Barter, Nicholls and Rye 10 ). Previous studies in our laboratory have shown that there is a significant age-related decrease in the antioxidant activity of HDL and in the capacity of HDL to mediate cholesterol efflux( Reference Khalil, Jay-Gerin and Fulop 4 , Reference Seres, Paragh and Deschene 11 , Reference Berrougui, Isabelle and Cloutier 12 ). However, to date, no studies have addressed the changes in the anti-inflammatory activity of HDL with ageing and the factors that may modulate this important anti-atherogenic property of HDL in the elderly.
The anti-inflammatory activity of HDL has been attributed to apoAI and phospholipids, including sphingosine-1-phosphate and sphingosyl-phosphorylcholine( Reference Baker, Rye and Gamble 13 , Reference Recalde, Ostos and Badell 14 ). However, there is strong evidence that this activity also involves paraoxonase 1 (PON1), which acts alone or in combination with other HDL-associated enzymes to inhibit or retard the inflammatory process( Reference Loued, Isabelle and Berrougui 15 , Reference Shih, Gu and Xia 16 ).
Human serum PON1 is primarily synthesised by the liver and is associated exclusively with HDL( Reference Deakin, Leviev and Gomaraschi 17 ). PON1 activity is inversely related to cardiovascular risks( Reference Mackness, Mackness and Durrington 18 – Reference Durrington, Mackness and Mackness 20 ). Shih et al. ( Reference Shih, Gu and Xia 16 ) eloquently showed that PON1 plays a role in the anti-atherogenic properties of HDL. HDL from PON1 knockout mice do not protect LDL against oxidation or reduce the amount or chemotactic activity of monocyte chemotactic protein-1, unlike HDL from wild-type mice( Reference Shih, Gu and Xia 16 ). PON1 has been reported to inhibit the induction of MCP1 in endothelial cells, probably due to its antioxidant activity( Reference Mackness, Hine and Liu 21 ). Marsillach et al. ( Reference Marsillach, Camps and Ferre 22 ) suggested that PON1 protects against liver inflammation mediated by monocyte chemotactic protein-1, while Watson et al. ( Reference Watson, Navab and Hama 23 ) suggested that PON1 possesses phospholipase-A2-like activity that allows it to hydrolyse oxidised phospholipids at the sn-2 position. PON1 also inhibits oxLDL-induced inflammation and reduces intracellular adhesion molecule (ICAM) expression on endothelial cells( Reference Loued, Isabelle and Berrougui 15 , Reference Shih, Gu and Xia 16 ).
There is a growing body of evidence, including results from prospective studies, indicating that reduced HDL-associated PON1 activity is predictive of vascular disease in humans( Reference Mackness, Durrington and McElduff 24 , Reference Robertson, Hawe and Miller 25 ). Low PON1 paraoxonase activity has been found in numerous pathological conditions associated with atherosclerosis, including type 1 and 2 diabetes, hypercholesterolaemia and the metabolic syndrome, as well as in elderly populations( Reference Seres, Paragh and Deschene 11 , Reference Deakin and James 26 – Reference Senti, Tomas and Anglada 28 ). All these conditions have a pro-inflammatory baseline state. The composition of HDL is altered during the acute-phase response of the innate immune system. In particular, acute-phase HDL differs from normal HDL in terms of its protective effect against atherosclerosis( Reference Yu, Yekta and Vakili 29 ).
Atherosclerosis is a disease with a multi-faced aetiology. Diet is one of the most important environmental factors influencing the progression of the disease. The Mediterranean diet, which has been used in the Mediterranean Basin for over 2000 years, is rich in cereals, vegetables, fruits and legumes, and is low in cholesterol and SFA. The main source of fat is virgin olive oil, especially first-press extra-virgin olive oil (EVOO), which retains important minor compounds that have anti-atherosclerotic properties( Reference Perona, Cabello-Moruno and Ruiz-Gutierrez 30 ). Considerable attention is being paid to the potential health benefits of olive oil. Human consumption of olive oil lowers major atherosclerotic risk factors by improving the lipoprotein profile, blood pressure, glucose metabolism and oxidative stress( Reference Perez-Jimenez, Alvarez de Cienfuegos and Badimon 31 ). Olive oil may exert its anti-atherosclerotic effect by increasing HDL levels( Reference Mata, Alvarez-Sala and Rubio 32 ). However, the effect of olive oil consumption on the atheroprotective properties of HDL (functionality of HDL) has not been investigated. The functionality of HDL may be as relevant to cardiovascular risk assessment as plasma HDL concentrations( Reference Stock 33 ). The main goals of the present study were to assess the anti-inflammatory properties of HDL as a function of ageing and the involvement of PON1 in this process, and to determine whether consuming EVOO for 12 weeks would improve the anti-inflammatory activity of HDL in both young and elderly volunteers.
Materials and methods
Chemicals
SDS, EDTA, bovine serum albumin and O, O-diethyl-O-p-nitrophenyl-phosphate (paraoxon) were from Sigma-Aldrich. Dialysis membranes were from Spectrum Medical Industries, Inc. Dulbecco's modified Eagle's medium and fetal bovine serum were from Wisent, Inc. Anti-CD54 monoclonal antibody (ICAM-1, clone 1A29) and anti-mouse IgG1 monoclonal antibody (clone 4639) were from BD Bioscience. All other chemicals were from Sigma-Aldrich. THP-1 (human acute monocytic leukaemia cell line) cells were from the American Type Culture Collection (ATCC). The EA.hy926 endothelial hybrid cell line was kindly provided by Dr C. J. Edgell (University of North Carolina, NC, USA). Roswell Park Memorial Institute (RPMI)-1640 medium was from Invitrogen Canada, Inc. EVOO was from Atlas Olive Oils.
Study procedure and extra-virgin olive oil supplementation
A total number of twenty healthy subjects (eleven men and nine women) were recruited and were divided into two groups; ten young (20–30 years) and ten elderly (65–85 years) subjects in each group. They were all healthy normolipidaemic non-smokers. None had clinical or laboratory signs of hypertension, inflammation or diabetes, and all had normal thyroid function test results. None was smoking, or taking medications or oral antioxidant supplements.
Participants were asked to consume 25 ml/d of EVOO in its raw state for 12 weeks. None of the participants followed any specific recommendation regarding diet or physical activity before the study. All subjects participated normally in all their daily activities without modifications throughout the study duration. Blood tests were performed at recruitment (T0; baseline) and after 12 weeks of EVOO consumption (T12).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the University Institute of Geriatrics of Sherbrooke (#2009/19). Written informed consent was obtained from all subjects.
Blood collection
After an overnight fast, 80 ml of blood samples, which provided approximately 35 ml of plasma, were collected from the volunteers in EDTA- (LDL and HDL purification) and heparin-containing tubes (PON1 purification) at T0 and T12. Plasma was separated by low-speed centrifugation (1500 g ), and 20 ml were used immediately to isolate lipoproteins (LDL and HDL). The remaining plasma (20 ml) was stored at − 80°C until used to purify PON1.
LDL and HDL isolation
EDTA-containing plasma samples (20 ml) obtained from the young and elderly donors were used within 1 h of collection to isolate LDL and HDL (at T0 and T12) using the method of Sattler et al. ( Reference Sattler, Mohr and Stocker 34 ). Briefly, LDL (1·019 < d< 1·063) and HDL (1·063 < d< 1·19) were separated by ultracentrifugation at 100 000 rpm for 2 h at 15°C using a TLA 100.4 rotor. The lipoprotein samples were placed in Spectrapor membrane tubing (12 000–14 000 exclusion limit; Spectrum Medical Industries) and dialysed extensively overnight at 4°C in 10 mm-sodium phosphate buffer (pH 7·0) with two changes of buffer. HDL and LDL concentrations are expressed as total protein concentrations, which were determined by spectrophotometry (595 nm) using the Bio-Rad Protein Assay as described by the manufacturer (Bio-Rad Laboratories).
LDL peroxidation and measurement of the basal oxidative status of HDL
Peroxidation was performed as previously described using transition metal ions as oxidising agents( Reference Khalil and Fulop 35 ). Briefly, 100 μg/ml of LDL were suspended in 10 mm-sodium phosphate buffer (pH 7) containing 10 μm-cupric sulphate. The suspension was incubated at 37°C for 16 h. The reaction was stopped by adding 200 μm-EDTA and cooling to 4°C. LDL peroxidation was determined by measuring conjugated diene formation by monitoring absorbance at 234 nm( Reference Pryor and Castle 36 ).
The basal oxidative status of HDL was determined by measuring the conjugated diene content and the electrophoretic mobility of HDL immediately after isolation.
Measurement of oxidative stress
Systemic oxidative stress was evaluated by the measurement of plasma carbonyl content, which was assayed as described by Levine et al. ( Reference Levine, Garland and Oliver 37 ). Briefly, the carbonyl content was determined by dinitrophenylhydrazine derivatisation and detected in trichloroacetic acid-precipitable materials by absorbance at 370 nm (ɛ = 22 000 per mcm).
Paraoxonase 1 purification
PON1 was purified from the plasma of each volunteer. Briefly, frozen heparin-containing plasma (20 ml) was defibrinated, and PON1 was purified using blue agarose (Cibacron Blue 3GA) as described previously( Reference Gan, Smolen and Eckerson 38 ), with some modifications. The defibrinated plasma samples were mixed with an equal volume of blue agarose that had been pre-equilibrated overnight with buffer A (20 mm-Tris–HCl, pH 8·0) containing 2 mm-CaCl2 and 4 m-NaCl. The mixtures were rinsed four times with 100 ml of buffer A containing decreasing concentrations of NaCl (4, 3, 2 and 1 m) and then twice with 100 ml of NaCl-free buffer A to reduce the ionic strength. The mixtures were then loaded in a column and the bound PON1 was released from the blue agarose using buffer A containing 0·1 % deoxycholic acid. The high activity fractions were pooled, dialysed and concentrated using a Centricon 30 microconcentrator (Amicon). The PON1 concentrate was then applied to a concanavalin A-sepharose (Sigma) column (15 cm/1 cm; Amicon Corporation) that had been equilibrated overnight with buffer B (25 mm-Tris–HCl, pH 7·4) containing 1 mm-CaCl2, 0·15 m-NaCl and 0·1 % (v/v) Triton X-100. The pooled fractions from the Cibacron Blue 3GA chromatography step were applied at a rate of 0·35 ml/min to the column. The column was washed with the same buffer to eliminate most of the impurities (minor amounts of apoAI and albumin remained)( Reference Valiyaveettil, Alamneh and Biggemann 39 ). The bound enzyme was eluted (1 ml fractions) using a linear gradient of 40 ml of buffer A and then 40 ml of buffer B containing 0·35 m-methyl α-mannopyranoside at 0·5 ml/min. The fractions with the highest PON1 activity were pooled and concentrated using a Centricon 30 microconcentrator, which also removed most of the contaminating concanavalin A fragments( Reference Rodrigo, Mackness and Durrington 40 ).
Paraoxonase genotype determination
PON1 R192Q genotypes were determined by PCR using a previously published protocol( Reference Mackness, Mackness and Arrol 41 ), with slight modifications. For the 192 polymorphism, sense primer 5′-TATTGTTGCTGTGGGACCTGAG-3′ and antisense primer 5′-CACG CTAAACCCAAATACATCTC-3′, which encompass the 192 polymorphic region of the human PON1 gene, were used. The PCR mixture contained 200 ng of DNA template, 0·5 μm of sense primer and 0·5 mm of antisense primer, 200 μm-dNTPs and 1 U of Taq DNA polymerase (New England Biolabs). DNA was denatured for 5 min at 95°C. The PCR protocol was as follows: forty-six denaturing cycles (1 min at 94°C), a 30 s annealing step at 61°C and a 1 min extension step at 72°C. The 99 bp PCR product was digested with 5 U of Alw l restriction endonuclease (New England Biolabs) for 4 h at 37°C. The digestion products were separated on 2 % agarose gels and visualised using SYBR Green (Sigma). The R-genotype (arginine) contains a single Alw l restriction site, which results in 66 and 33 bp products. The Q-genotype (glutamine) is not cut, which allows the PON1 192 genotype to be determined.
Paraoxonase 1 and arylesterase activities and paraoxonase 1 plasma concentrations
PON1 paraoxonase activity was measured by monitoring the increase in absorbance at 412 nm using paraoxon (O, O-diethyl-O-p-nitrophenylphosphate; Sigma) as the substrate, as already described( Reference Seres, Paragh and Deschene 11 ).
PON1 paraoxonase and arylesterase activities were measured by monitoring the increase in absorbance at 270 nm using phenylacetate as the substrate, as already described( Reference Seres, Paragh and Deschene 11 ).
Plasma PON1 concentrations were measured using ELISA kits (Uscn Life Science, Inc.). Absorbance at 405 nm was measured using a microplate reader (Bio-Rad), and a calibration curve (3·12–200 ng/ml) was used to determine PON1 protein concentrations.
Cell cultures
The EA.hy926 endothelial hybrid cell line was used to measure the ICAM-1 expression. EA.hy926 cells, which are the most similar of all immortalised human endothelial cell lines to human umbilical vein endothelial cells, were used to avoid the variability and time and effort associated with primary isolations( Reference Lidington, Moyes and McCormack 42 ). EA.hy926 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10 % fetal bovine serum, 5 mm-hypoxanthine, 20 μm-aminopterin, 0·8 mm-thymidine and 100 μg/ml of penicillin/streptomycin.
Human THP-1 monocytes were cultured in RPMI-1640 medium in a 5 % CO2 atmosphere at 37°C. The medium was supplemented with 10 % heat-inactivated fetal bovine serum, 10 % pyruvate sodium, 1·5 mg/ml of glucose and 100 U/ml of penicillin.
Expression of intracellular adhesion molecule 1
The expression of the adhesion molecule ICAM-1 on EA.hy926 cells was analysed by flow cytometry. The cells were washed with PBS before being trypsinised for 2 min at 37°C. Trypsin was inactivated by adding medium containing 10 % fetal bovine serum. The cells were centrifuged (2 min, 4000 rpm), and the supernatant was discarded. The cells were incubated with a phycoerythrin-conjugated anti-ICAM-1 monoclonal antibody (1 μg/106 cells) for 15 min at 25°C. They were then washed with PBS and analysed using a FACSCalibur instrument (Becton Dickinson). Data were analysed using CellQuest software (BD Biosciences).
Chemotaxis assay
THP-1 chemotaxis was measured using a modified Boyden chamber chemotaxis assay. Assays were performed in duplicate using 200 μl of cells in the upper chamber. THP-1 monocytes were suspended at a concentration of 2 × 106 cells/ml in chemotaxis buffer (RPMI-1640 medium without phenol red). The two chambers were separated by a 5 μm pore size polycarbonate filter (Osmonics). Basal migration (negative control) was measured in the absence of chemoattractant (medium alone). Chemotaxis was assessed in the presence of 10 nm-N-formyl-methionine-leucine-phenylalanine. After a 2 h incubation at 37°C in a 5 % CO2 atmosphere, the chambers were disassembled and the upper side of the filter was scraped free of cells. Cells on the lower side of the filter were removed using 10 mm-EDTA and were combined with the cells that had migrated into the lower chamber. The cells were centrifuged, resuspended in 150 μl PBS and counted by a flow cytometer. Events were acquired over a fixed period using CellQuest software (BD Biosciences).
Statistical analysis
Values are means and standard deviations. Mean values were compared using a one-way ANOVA followed by a Bonferroni or Mann–Whitney test. P values less than or equal to 0·05 were considered to be significant. Statistical analyses were performed using GraphPad Prism software, version 5.0 (GraphPad Software, Inc.).
Results
The twenty volunteers (eleven males and nine females) were distributed into two groups as a function of age (young, n 10, mean age 29 (sd 5·41) years; elderly, n 10, mean age 71·60 (sd 4·62) years). The baseline demographic and anthropometric characteristics of the volunteers are summarised in Table 1. The two age groups had comparable normal BMI (23·85 (sd 4·07) and 25·28 (sd 4·27) kg/m2 for the young and elderly volunteers, respectively). While total cholesterol, LDL and plasma glucose values were normal for the two groups, they were slightly but significantly higher in the elderly volunteers than in the young volunteers (Table 1). In addition, at T0, the elderly volunteers had significantly higher diastolic and systolic blood pressures than the young volunteers. There were no significant differences between the two groups for the other biochemical and clinical parameters. Plasma tyrosol and hydroxytyrosol contents were measured and showed a small (not statistically significant) increasing trend after 12 weeks of EVOO consumption (results not shown).
Table 1 Anthropometric and biochemical characteristics of the volunteers at baseline and after 12 weeks of extra-virgin olive oil (EVOO) consumption (Mean values and standard deviations)
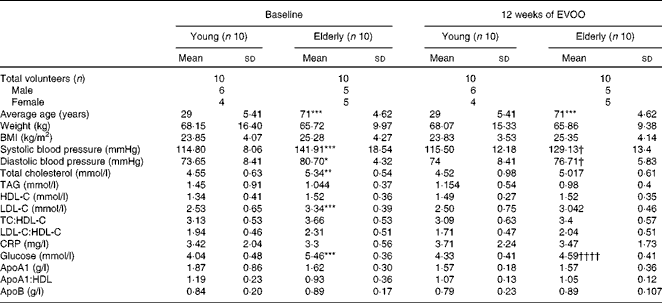
HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; TC, total cholesterol; CRP, C-reactive protein.
Mean values were significantly different for the elderly volunteers compared with the young volunteers at baseline: * P< 0·05, ** P< 0·01, *** P< 0·001 (Mann–Whitney test).
Mean values were significantly different for the elderly volunteers after 12 weeks of EVOO consumption compared with the elderly volunteers at baseline: † P< 0·05 and †††† P< 0·0001 (Mann–Whitney test).
Effect of ageing on the anti-inflammatory activity of HDL
The anti-inflammatory activity of HDL is due in part to the capacity to reduce the expression of adhesion molecules such as ICAM-1 on endothelial cells. Initial experiments were carried out to determine the basal anti-inflammatory activity of HDL as a function of age. HDL from young (Y-HDL) and elderly (E-HDL) volunteers (200 μg/ml) were incubated for 16 h with EA.hy926 cells. Cells exposed to 10 ng/ml of TNF-α were used as a positive control. TNF-α induced an 89·90 (sd 4·03) % (P< 0·001) increase in ICAM-1 expression, whereas HDL alone induced a significant decrease in ICAM-1 expression (Fig. 1(a)). Interestingly, Y-HDL had higher anti-inflammatory activity, reducing ICAM-1 expression by 71·28 (sd 4·26) % compared with 55·78 (sd 1·75) % for E-HDL (P< 0·05).
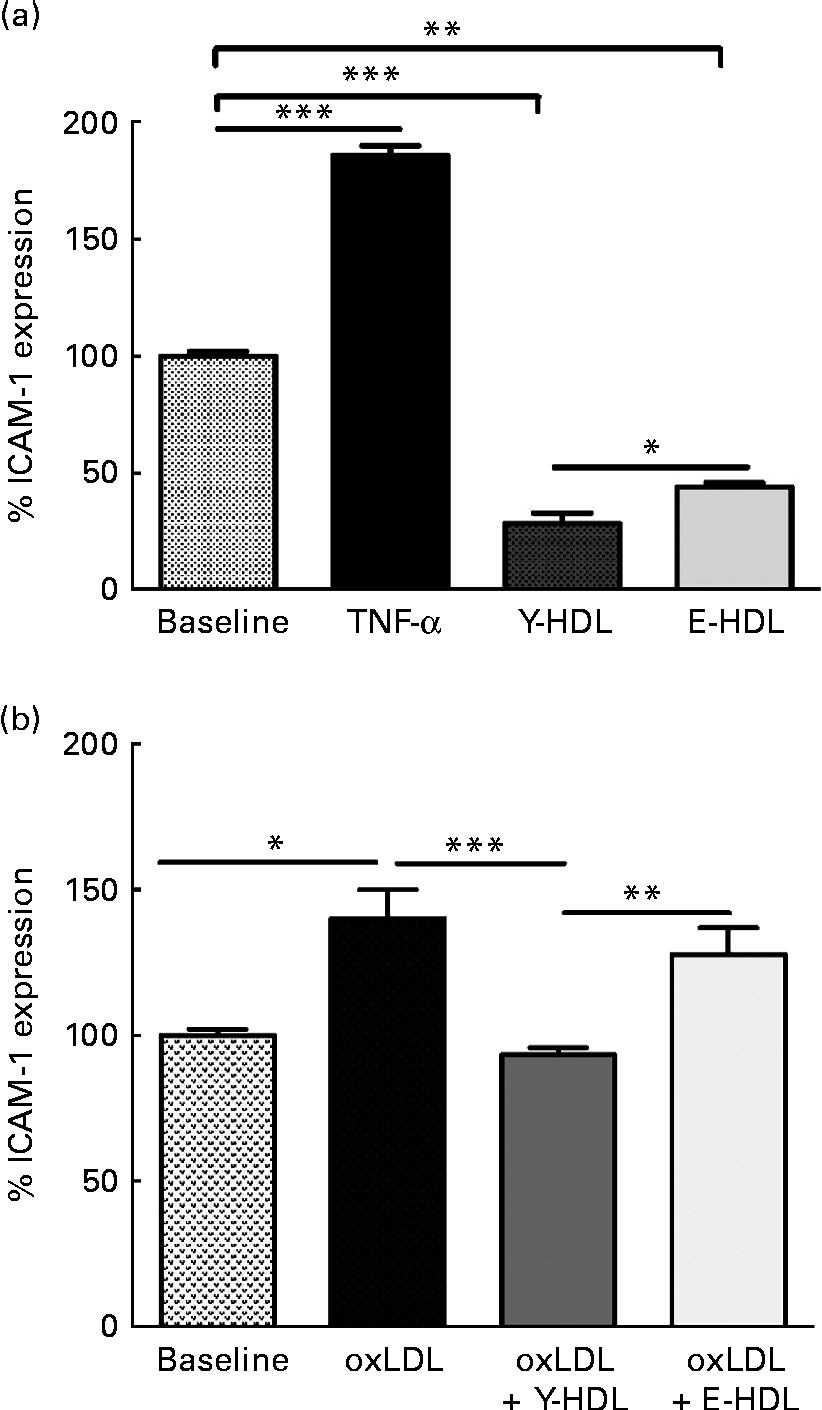
Fig. 1 Age-related decrease in the capacity of HDL to reduce intracellular adhesion molecule 1 (ICAM-1) expression on endothelial cells. HDL (200 μg/ml) isolated from healthy young (Y-HDL) and elderly (E-HDL) volunteers were incubated with EA.hy926 endothelial cells for 16 h. Cells incubated with 10 ng/ml of TNF-α were used as a positive control. ICAM-1 expression was assessed by fluorescence-activated cell sorter analysis. The anti-inflammatory effect of HDL was measured in the (a) absence or (b) presence of 100 μg/ml of oxidised LDL (oxLDL). Values are means, with standard deviations represented by vertical bars. Mean values were significantly different: * P< 0·05, ** P< 0·01, *** P< 0·001 (one-way ANOVA followed by Bonferroni multiple comparison post-test).
We also investigated the anti-inflammatory activity of HDL in the presence of oxLDL. oxLDL (100 μg/ml) induced a 40·14 (sd 9·86) % (P< 0·05) increase in ICAM-1 expression compared with EA.hy926 cells incubated in the absence of oxLDL (Fig. 1(b)). However, 200 μg/ml of HDL significantly reduced the pro-inflammatory effect of oxLDL. The anti-inflammatory effect was age-dependent. In addition, Y-HDL had greater anti-inflammatory activity than E-HDL, reducing ICAM-1 expression by 46·28 (sd 2·5) % (P< 0·001), while E-HDL had no significant effect (Fig. 1(b)).
In an attempt to determine the factors involved in the age-related decrease in the anti-inflammatory activity of HDL, we assessed the oxidative modifications to the lipid and protein fractions of Y- and E-HDL by measuring conjugated diene formation and electrophoretic mobility. The conjugated diene content (Fig. 2(a)) and electrophoretic mobility (Fig. 2(b)) of E-HDL were significantly higher than those of Y-HDL.
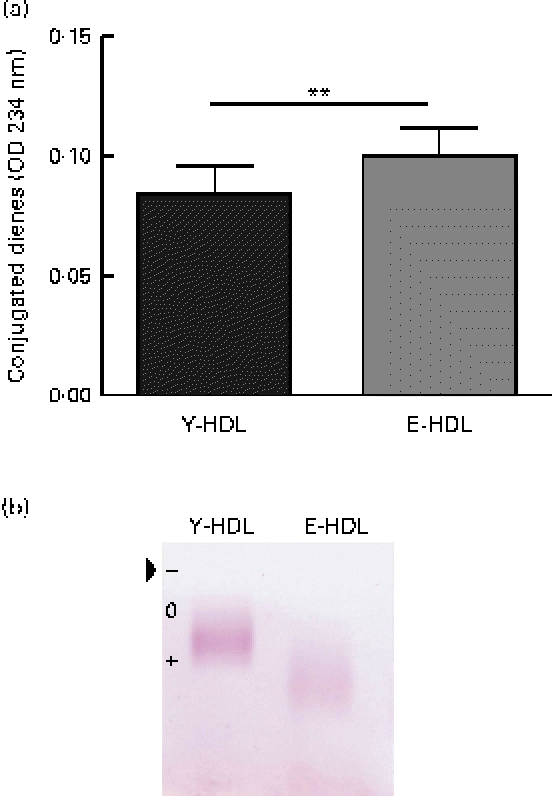
Fig. 2 Measurement of basal oxidative damage to HDL. (a) Conjugated diene levels were assessed by measuring the optical density (OD) at 234 nm. ** Mean values were significantly different (P< 0·01). (b) Electrophoretic mobility was assessed by migrating young-HDL (Y-HDL) and elderly-HDL (E-HDL) on 0·6 % agarose gels and staining with Fat Red 7B in 95 % methanol. The arrow indicates the starting point. Relative electrophoretic mobilities were determined by comparing the electrophoretic mobility of E-HDL to Y-HDL at baseline. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Effect of ageing on the anti-inflammatory activity of paraoxonase 1
The anti-inflammatory activity of HDL has been attributed, in part, to the activity of PON1( Reference Shih, Gu and Xia 16 ). To elucidate the mechanism responsible for the decrease in the anti-inflammatory activity of E-HDL, we investigated the effect of ageing on the anti-inflammatory activity of PON1. Plasma PON1 R192Q genotypes, paraoxonase and arylesterase activities, and PON1 plasma concentrations were determined for all the volunteers. The PON1 genotypes (R192Q) were equally distributed between the two age groups (Table 2). We observed no significant differences in PON1 paraoxonase and PON1 paraoxonase and arylesterase activities or PON1 plasma concentrations between the two age groups.
Table 2 Paraoxonase 1 (PON1) R192Q genotypes, activities and plasma concentrations of the volunteers at baseline and after 12 weeks of extra-virgin olive oil (EVOO) consumption (Mean values with their upper and lower limits)
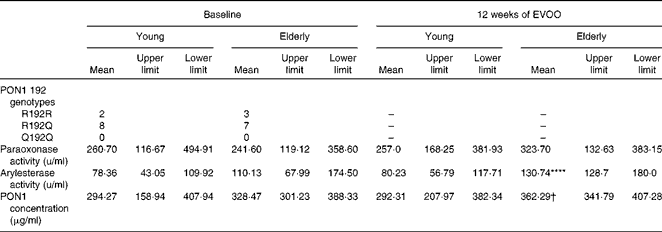
**** Mean value was significantly different compared with young volunteers at baseline (P< 0·0001).
† Mean value was significantly different compared with elderly volunteers at baseline (P< 0·05).
PON1 was purified from the plasma of all the young (Y-PON1) and elderly (E-PON1) volunteers and was used at the same protein concentration (40 μg protein/ml). The anti-inflammatory activities of the purified PON1 samples were assessed by their capacity to inhibit or reduce ICAM-1 expression on EA.hy926 cells. The cells were incubated with 100 μg/ml of oxLDL alone, 100 μg/ml of oxLDL and 200 μg/ml of pooled HDL, or PON1-enriched HDL (40 μg/ml of PON1). Enriching HDL with PON1 significantly increased the anti-inflammatory effect (Fig. 3(a)). However, Y-PON1 induced the highest anti-inflammatory activity, with a 32·64 (sd 2·63) % (P< 0·01) decrease in ICAM-1 expression compared with pooled HDL alone, while E-PON1-enriched HDL had no significant effect.
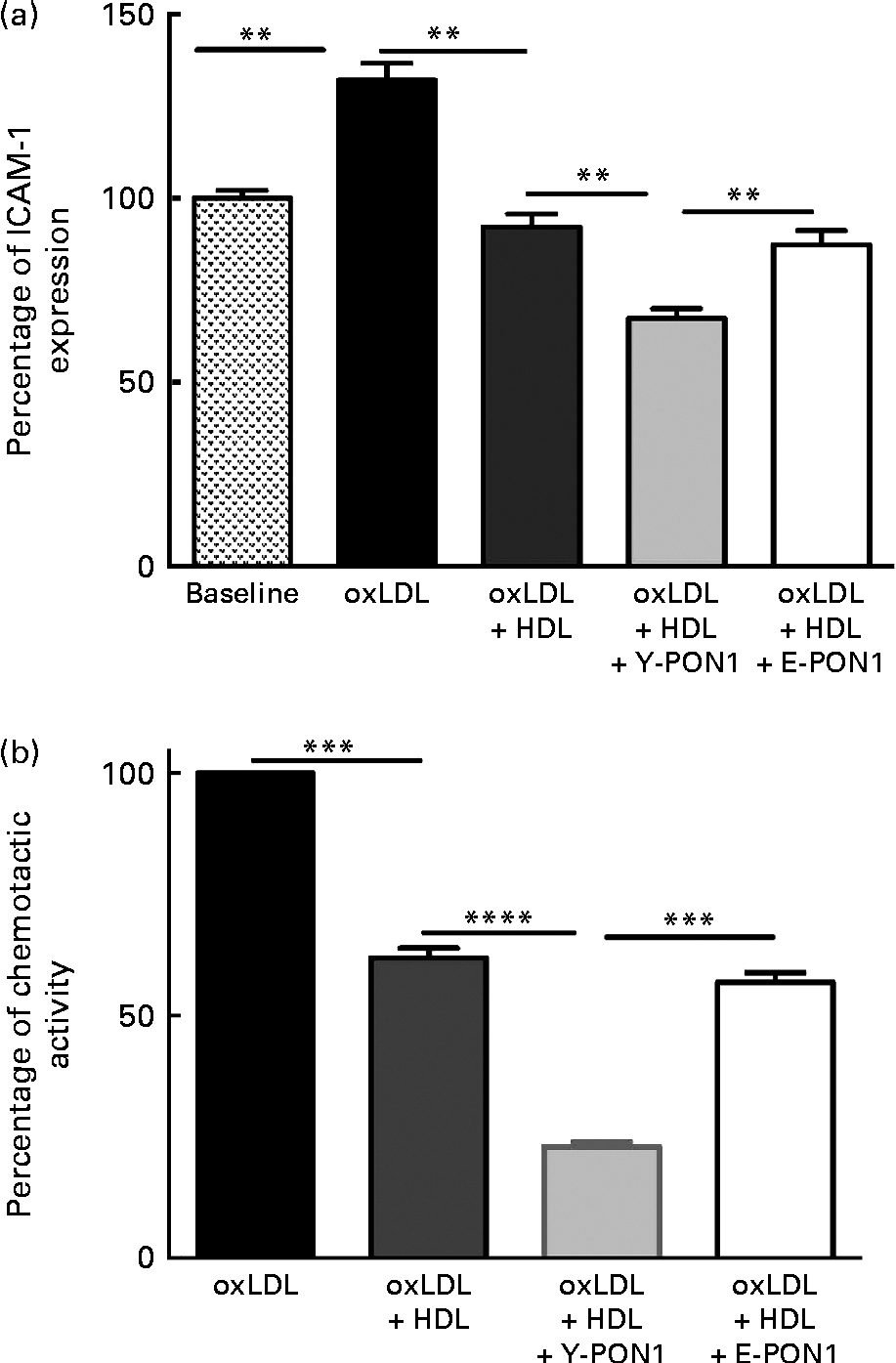
Fig. 3 Anti-inflammatory activity of paraoxonase 1 (PON1) decreases with age. The anti-inflammatory activity of PON1 was assessed (a) by measuring intracellular adhesion molecule 1 (ICAM-1) expression on EA.hy926 endothelial cells and (b) by measuring THP-1 (human acute monocytic leukaemia cell line) monocyte chemotaxis using a modified Boyden chamber chemotactic assay. PON1 purified from the plasma of healthy young (Y-PON1) and elderly (E-PON1) volunteers was used at a concentration of 40 μg protein/ml. EA.hy926 cells were used 2 d post-confluence and were incubated for 16 h with 100 μg/ml of oxidised LDL (oxLDL) alone or in the presence of 200 μg/ml of oxLDL and HDL or PON1-enriched HDL. For the chemotactic measurements, THP-1 monocytes were suspended at a concentration of 2 × 106 cells/ml in chemotactic buffer (RPMI-1640 medium without phenol red). Basal migration (negative control) was measured in the absence of chemoattractant (medium alone). Chemotaxis was assessed in the presence of 10 nm-N-formyl-methionine-leucine-phenylalanine. Values are means, with their standard deviations represented by vertical bars. Mean values were significantly different: ** P< 0·01, *** P< 0·001, **** P< 0·0001 (one-way ANOVA followed by Bonferroni multiple comparison post-test).
To confirm the anti-inflammatory activity of PON1 and the age-related decrease in PON1 activity, we investigated its capacity to reduce macrophage migration and chemotaxis by incubating THP-1 monocytes alone (basal condition) or with oxLDL in the absence or presence of pooled HDL for 2 h at 37°C. In another series of experiments, Y- and E-PON1 samples were incubated separately for 4 h with HDL to produce PON1-enriched HDL. The samples were then supplemented with oxLDL and their chemotactic activity was assessed. OxLDL (100 μg/ml) significantly increased monocyte migration (Fig. 3(b)) while 200 μg/ml of HDL reduced monocyte migration by 38·14 (sd 9·59) % (P< 0·05). Interestingly, while the ability of HDL to inhibit THP-1 chemotaxis was significantly improved by enriching HDL with PON1, Y-PON1 was more effective at reducing THP-1 chemotaxis ( − 62·84 (sd 13·16) %). No significant effect was observed when oxLDL was incubated with E-PON1-enriched HDL compared with oxLDL incubated with HDL alone (Fig. 3(b)).
Effect of extra-virgin olive oil consumption on biochemical and clinical parameters
In the second part of the present study, we investigated the effect of 12 weeks of EVOO consumption on the anti-inflammatory activity of HDL. As shown in Table 1, 12 weeks of EVOO consumption did not induce significant changes in the lipid profile (LDL-cholesterol, HDL-cholesterol, total cholesterol and TAG) or other clinical parameters of either age group. However, plasma glucose concentrations (5·46 (sd 0·36) mmol/l at T0 v. 4·59 (sd 0·41) mmol/l at T12, P< 0·0001) and systolic (141·91 (sd 18·54) v. 129·13 (sd 13·4) mmHg, P< 0·05) and diastolic (80·70 (sd 4·32) v. 76·71 (sd 5·83) mmHg, P< 0·05) blood pressures in the elderly group were significantly reduced at T12.
There was no significant change in PON1 paraoxonase activity in either group after 12 weeks of EVOO consumption, although a slight increase was observed in the elderly group. There was a significant increase in arylesterase activity and PON1 plasma concentrations in the elderly group at T12 (Table 2).
Extra-virgin olive oil consumption increases the anti-inflammatory activity of HDL and reduces oxidative damage to HDL
The effect of EVOO consumption on the anti-inflammatory activity of HDL was assessed by measuring ICAM-1 expression on EA.hy926 cells in the presence of HDL obtained at T0 and T12. The results from confluent EA.hy926 cells incubated with 100 μg/ml of oxLDL alone or in combination with 200 μg/ml of Y-HDL or E-HDL obtained at T12 were compared with the results obtained at T0. While used at the same concentration (200 μg/ml), Y- and E-HDL obtained at T12 had a greater anti-inflammatory effect than Y- and E-HDL obtained at T0. The anti-inflammatory effect of HDL, upon 12 weeks of EVOO intake, was more improved in E-HDL than Y-HDL, as reflected through the ICAM expression reduction by 32.2 v. 18.5%, respectively. At T12, the anti-inflammatory activity of E-HDL was the same as that of Y-HDL at T0 (Fig. 4(a)). These results were confirmed by experiments comparing the capacity of Y- and E-HDL at T0 and T12 to reduce monocyte chemotaxis (Fig. 4(b)).
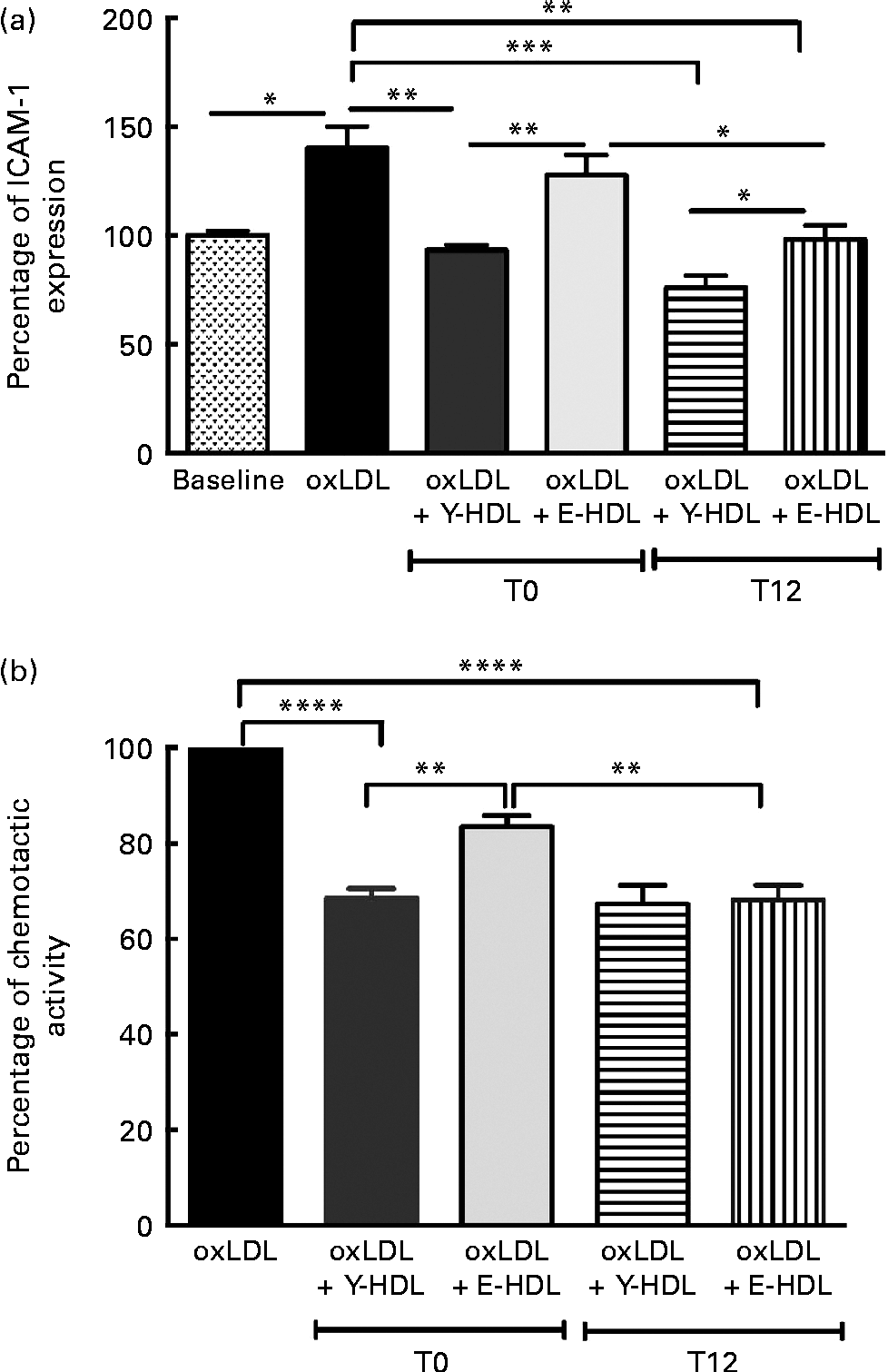
Fig. 4 Extra-virgin olive oil (EVOO) consumption improves the anti-inflammatory activity of HDL and the ability of HDL to reduce THP-1 (human acute monocytic leukaemia cell line) monocyte chemotaxis. (a) The anti-inflammatory activity of HDL was assessed by measuring intracellular adhesion molecule 1 (ICAM-1) expression on EA.hy926 endothelial cells and (b) by measuring THP-1 monocyte chemotaxis using a modified Boyden chamber chemotactic assay. THP-1 monocytes were suspended at a concentration of 2 × 106 cells/ml in chemotactic buffer (RPMI-1640 medium without phenol red). Basal migration (negative control) was measured in the absence of chemoattractant (medium alone). Chemotaxis was assessed in the presence of 10 nm-N-formyl-methionine-leucine-phenylalanine. EA.hy926 cells and THP-1 monocytes were incubated for 16 h with 100 μg/ml of oxidised LDL (oxLDL) alone or in the presence of 200 μg/ml of young-HDL (Y-HDL) or elderly-HDL (E-HDL). Y- and E-HDL were isolated from the plasma of the volunteers at baseline (T1) and after 12 weeks of EVOO consumption (T12). Values are means, with their standard deviations represented by vertical bars. Mean values were significantly different: * P< 0·05, ** P< 0·01, *** P< 0·001, **** P< 0·0001 (one-way ANOVA followed by Bonferroni multiple comparison post-test).
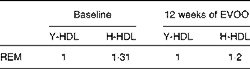
We also measured the oxidative damage to Y- and E-HDL at T12 and compared the results with those obtained at T0. The 12 weeks of EVOO consumption induced a significant decrease in the conjugated diene content of E-HDL, while no significant changes were observed for Y-HDL (Fig. 5(a)). In addition, 12 weeks of EVOO consumption reduced the electrophoretic mobility of E-HDL but had no effect on Y-HDL (Fig. 5(b); Table 3). These results were confirmed by the determination of systemic oxidative status as measured by the plasma carbonyl content. Indeed, 12 weeks of EVOO content decreased significantly the plasma carbonyl content for both young and elderly volunteers (Fig. 5(c)).
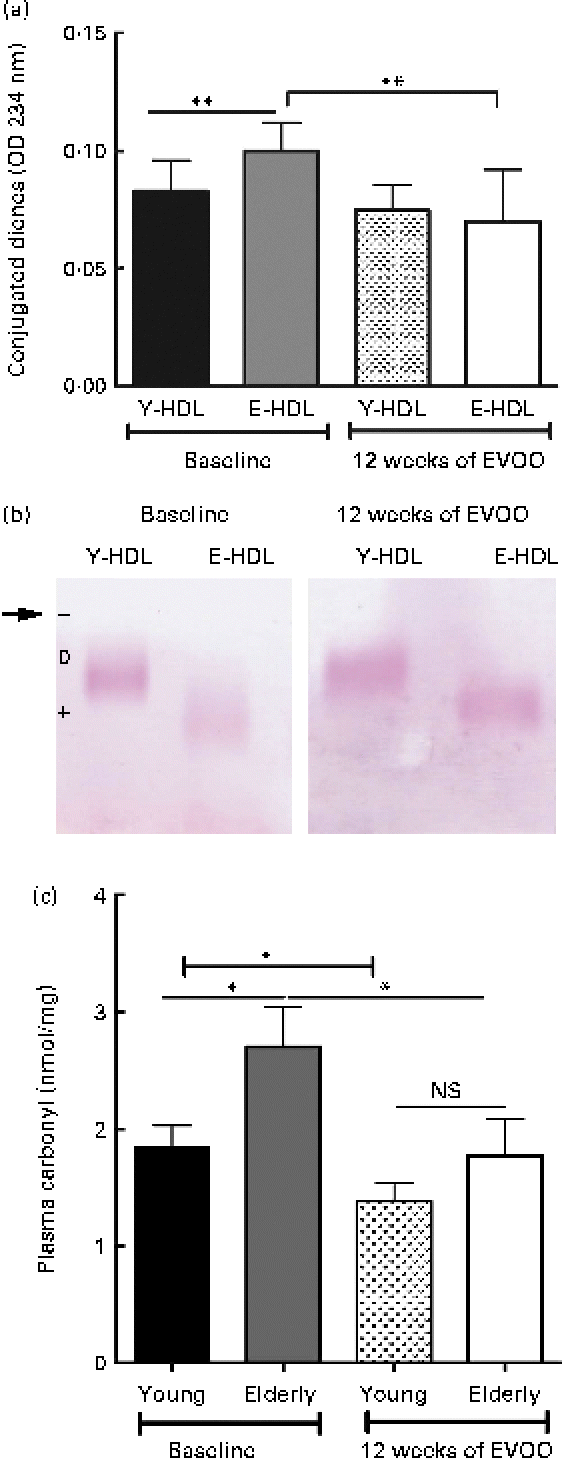
Fig. 5 Effect of 12 weeks of extra-virgin olive oil (EVOO) consumption on oxidative modifications to HDL. (a) Conjugated diene formation was assessed by optical density (OD) measurements at 234 nm. (b) Electrophoretic mobility was assessed by separating young-HDL (Y-HDL) and elderly-HDL (E-HDL) on 0·6 % agarose gels and staining with Fat Red 7B in 95 % methanol. The arrow indicates the starting point. Relative electrophoretic mobilities were assessed by comparing the electrophoretic mobility of E-HDL with Y-HDL at baseline and after 12 weeks of EVOO consumption (Table 3). (c) Systemic oxidative stress status was evaluated by the measurement of plasma carbonyl content. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Table 3 Relative electrophoretic mobility (REM) of elderly-HDL (E-HDL) compared with young-HDL (Y-LDL) at baseline and after 12 weeks of extra-virgin olive oil (EVOO) consumption
Extra-virgin olive oil increases the anti-inflammatory activity of purified paraoxonase 1
PON1 was purified from plasma samples from all the volunteers at T0 and T12. HDL isolated from pooled plasma from the young and elderly volunteers were enriched with 40 μg/ml of E- or Y-PON1. Inflammation was assessed by measuring ICAM-1 expression on EA.hy926 cells, and the results were compared with ICAM-1 expression in the presence of HDL alone or HDL enriched with E-PON1 obtained at T0. Enriching HDL with E-PON1 isolated from the plasma of the elderly volunteers at T0 did not increase the anti-inflammatory activity of HDL (Fig. 6), while E-PON1 obtained at T12 significantly increased the anti-inflammatory activity of HDL as measured by the significant reduction in ICAM-1 expression compared with HDL alone or HDL enriched with E-PON1 obtained at T0 (95·21 (sd 3·15), 140·66 (sd 8·15) and 144·86 (sd 9·36) %, respectively, P< 0·05). The 12 weeks of EVOO consumption thus increased the anti-inflammatory activity of E-PON1 by approximately 32·31 % (P< 0·05). Interestingly, there was no significant change between T0 and T12 in the ability of Y-PON1-enriched HDL to reduce ICAM-1 expression (results not shown).

Fig. 6 Effect of 12 weeks of extra-virgin olive oil (EVOO) consumption on the anti-inflammatory activity of paraoxonase 1 (PON1). The anti-inflammatory activity of PON1 was assessed by measuring intracellular adhesion molecule 1 (ICAM-1) expression on EA.hy926 endothelial cells. PON1 was isolated from the elderly (E-PON1) volunteers at baseline (T1) and after 12 weeks of EVOO consumption (T12). EA.hy926 cells were used 2 d post-confluence and were incubated for 16 h with 100 μg/ml of oxidised LDL (oxLDL) alone or with HDL enriched with E-PON1 (40 μg/ml) obtained at T1 and T12. Values are means, with their standard deviations represented by vertical bars. Mean values were significantly different: ** P< 0·01, **** P< 0·0001 (one-way ANOVA followed by Bonferroni multiple comparison post-test).
Discussion
A number of epidemiological studies have confirmed that there is an association between the Mediterranean diet and a reduction in CVD( Reference Sofi, Cesari and Abbate 43 ), and have attributed this beneficial effect to the high intake of olive oil( Reference Gjonca and Bobak 44 ). The consumption of large amounts of olive oil decreases TNF-α levels and reduces systemic inflammation( Reference Papageorgiou, Tousoulis and Psaltopoulou 45 ). However, the effect of olive oil on the anti-inflammatory activity of HDL has never been investigated. The main goals of the present study were to investigate the effect of ageing on the anti-inflammatory activity of HDL and to determine whether 12 weeks of EVOO consumption could improve this activity.
HDL inhibit the cytokine-induced expression of endothelial cell adhesion molecules (ICAM-1, vascular cell adhesion molecule 1 and E-selectin) both in vitro and in models of acute inflammation( Reference Cockerill, Rye and Gamble 46 , Reference Cockerill, Huehns and Weerasinghe 47 ). The present results showed that there is a significant decrease in the anti-inflammatory activity of HDL in the elderly volunteers compared with the young volunteers and that the decrease is independent of HDL concentrations. Morgantini et al. ( Reference Morgantini, Natali and Boldrini 48 ) showed that the anti-inflammatory activity of HDL is impaired in type 2 diabetes and attributed this alteration to oxidative stress conditions that may be induced by hyperglycaemia. The formation of conjugated dienes and the increase in the electrophoretic mobility of HDL as well as the carbonyl content in the plasma from the elderly volunteers indicated that there is an increase in oxidative damage to the lipid and/or protein fractions of HDL, which may occur as a result of oxidative stress conditions that develop with ageing.
Previous studies have attributed the inflammatory activity of HDL principally to apoAI and sphingosine-1-phosphate( Reference Kimura, Tomura and Mogi 49 ). However, animal studies have shown that a deficiency in PON1 predisposes to vascular inflammation( Reference Shih, Gu and Xia 16 ). These results point to an important role for PON1 in the anti-inflammatory activity of HDL( Reference Ng, Chu and Esposito 50 ). PON1, a lactonase synthesised by the liver, is bound exclusively to HDL in the bloodstream. This enzyme is thought to degrade oxidised phospholipids and to play an important role as an antioxidant and anti-inflammatory molecule. Several studies have demonstrated that PON1 paraoxonase activity is significantly reduced in hypercholesterolaemia, diabetes mellitus, chronic renal failure and cardiac diseases( Reference Seres, Paragh and Deschene 11 , Reference Rasic-Milutinovic, Popovic and Perunicic-Pekovic 51 – Reference Tomas, Senti and Garcia-Faria 55 ). We previously demonstrated that this activity also decreases with ageing and showed that there is a link between the paraoxonase and antioxidant activities of PON1( Reference Seres, Paragh and Deschene 11 , Reference Jaouad, Milochevitch and Khalil 56 ).
The mechanism of PON1 involvement in the anti-inflammatory activity of HDL has not been clearly established. PON1 has been reported to inhibit MCP1 induction in endothelial cells, probably due to its antioxidant activity( Reference Mackness, Hine and Liu 21 ). Marsillach et al. ( Reference Marsillach, Camps and Ferre 22 ) suggested that PON1 protects against liver inflammation mediated by monocyte chemotactic protein-1, while Watson et al. ( Reference Watson, Navab and Hama 23 ) suggested that PON1 possesses phospholipase-A2-like activity that allows it to hydrolyse oxidised phospholipids at the sn-2 position. A number of studies have indicated that the anti-inflammatory activity of HDL is associated with the ability of PON1 to hydrolyse the oxidised phospholipid constituents of oxLDL and oxidised HDL( Reference Ahmed, Ravandi and Maguire 57 ). We recently demonstrated that PON1 inhibits oxidised lipid-induced ICAM-1 expression on endothelial cells by hydrolysing oxidised phospholipids and that this effect is dependent on its interaction with other HDL-associated enzymes( Reference Loued, Isabelle and Berrougui 15 ). The results reported here show that enriching HDL with PON1 significantly increases the anti-inflammatory activity of HDL. Interestingly, the capacity of purified E-PON1 to modulate the anti-inflammatory activity of HDL was lower than that of Y-PON1. This result confirmed that PON1 plays a major role in regulating the anti-inflammatory activity of HDL and that the age-related decrease in the anti-inflammatory activity of HDL may be due to a reduction in the activity of PON1. We observed no age-related changes in the enzymatic activity of PON1 or its plasma concentration or in the level of apoA1, an activator of PON1, suggesting that the decrease in the anti-inflammatory activity of PON1 in the elderly volunteers may be caused by oxidative modifications to PON1 that affect its anti-inflammatory activity, which may explain the reduction in the capacity of PON1 to modulate the anti-inflammatory activity of HDL. Previous studies have shown that PON1 loses its enzymatic and antioxidant activities in oxidative stress conditions( Reference Jaouad, Milochevitch and Khalil 56 , Reference Jaouad, de Guise and Berrougui 58 ). Garin et al. ( Reference Garin, Kalix and Morabia 59 ) also showed that oxidative stress conditions induce a significant decrease in PON1 activity, probably due to the displacement of PON1 from HDL. Van Lenten et al. ( Reference Van Lenten, Hama and de Beer 60 ) demonstrated that the alterations to the anti-inflammatory activity of HDL during acute-phase immune responses are due to the displacement of HDL-associated proteins. Moreover, we previously demonstrated that the age-related decrease in the antioxidant activity of HDL is due to an alteration to the active site of PON1( Reference Jaouad, Milochevitch and Khalil 56 , Reference Jaouad, de Guise and Berrougui 58 , Reference Mehdi and Rizvi 61 ). The increase in oxidative damage to HDL, as measured by the formation of conjugated dienes and the change in electrophoretic mobility, confirms the presence of oxidative stress conditions that may induce the oxidation of PON1 and contribute to the alteration of the anti-inflammatory activity of HDL. While it is not known whether oxidative modifications to PON1 occur, oxidative damage to the protein fraction of HDL, as shown by the increase in electrophoretic mobility, may indicate that PON1 is also modified during ageing.
There is a growing scientific consensus that antioxidants, particularly the polyphenolic forms, may help lower the incidence of diseases such as certain cancers as well as CVD and neurodegenerative disease. A number of studies have reported that the high polyphenol content of EVOO is responsible for its anti-inflammatory activity( Reference Bogani, Galli and Villa 62 ). This beneficial effect is mediated by preventing the production of inflammatory cytokines and by inhibiting the production of adhesion molecules that activate endothelial cells( Reference Papageorgiou, Tousoulis and Psaltopoulou 45 , Reference Bogani, Galli and Villa 62 ). The present results showed that EVOO consumption also improves the anti-inflammatory activity of HDL. Interestingly, this effect was significant only for E-HDL.
In addition to its high MUFA content, principally oleic acid, EVOO contains other biologically active substances, including α-tocopherols, β-carotene, sterols, terpene, squalene and phenolic compounds( Reference Konstantinidou, Covas and Munoz-Aguayo 63 – Reference Covas, de la Torre and Farre-Albaladejo 65 ). The strong antioxidant nature of phenolic compounds has an anti-atherogenic effect, protecting lipids, especially lipoproteins, against oxidation( Reference Berrougui, Cloutier and Isabelle 66 ). This is in agreement with the present results showing that EVOO consumption resulted in a significant decrease in oxidative damage to the lipid and protein fractions of E-HDL as shown by the changes in conjugated diene content, electrophoretic mobility and plasma carbonyl measurement. The anti-inflammatory activity of PON1 also increased following the consumption of EVOO, especially in E-PON1. This suggested that the polyphenols in EVOO protect the protein fraction of HDL from oxidative damage and improve the anti-inflammatory activity of E-PON1.
EVOO only induced a significant increase in the arylesterase activity of E-PON1. The hydrolysis of phenylacetate, the substrate used to assay arylesterase activity, did not depend on the polymorphic form of PON1. The arylesterase activity of PON1 is considered to correspond to the concentration of the enzyme( Reference Connelly, Maguire and Picardo 67 ). The present results showed that there is a significant increase in PON1 paraoxonase and arylesterase activity and plasma PON1 concentrations together with a lower conjugated diene content in the HDL of the elderly volunteers after 12 weeks of EVOO consumption. The increase in PON1 paraoxonase and arylesterase activity and PON1 plasma concentrations may be due to the polyphenols in EVOO and may explain the improvement in the functionality of HDL in the elderly group. Some flavonoids, such as quercetin and catechin, increase serum PON1 activity in mice( Reference Fuhrman and Aviram 68 ) due to their antioxidant properties. Noll et al. ( Reference Noll, Hamelet and Matulewicz 69 ) showed that red wine polyphenol extracts increased hepatic PON1 gene expression and hepatic and plasma PON1 activities in a murine model of hyperhomocysteinaemia.
The increase in the anti-inflammatory activity of HDL following the consumption of EVOO by the elderly volunteers was independent of plasma HDL concentrations, indicating that a polyphenol-rich dietary supplement can improve the functionality of HDL and that polyphenols are as important, if not more so, than serum HDL concentrations in determining the atheroprotective capacity of HDL( Reference Sviridov, Mukhamedova and Remaley 70 ). Several studies, including the present study, have shown that the atheroprotective effect of HDL, especially in terms of antioxidant activity and cholesterol efflux, decreases significantly with ageing( Reference Berrougui, Isabelle and Cloutier 12 , Reference Jaouad, de Guise and Berrougui 58 ). The results of the present study also showed that the anti-inflammatory activity of HDL is lower in elderly individuals and that this decrease is due principally to the oxidative stress conditions that characterise the ageing process. This confirms the assertion that the beneficial effect of EVOO consumption on the anti-inflammatory activity of HDL is mediated by its ability to reduce oxidative stress conditions and HDL-associated oxidative damage.
It is noteworthy that, in addition to its beneficial effect on the anti-inflammatory activity of E-HDL, 12 weeks of EVOO consumption significantly reduced blood glucose concentrations and systolic and diastolic blood pressures in the elderly volunteers. These results are in agreement with previous studies showing that olive oil consumption has a beneficial effect on the blood pressure of both normotensive and hypertensive individuals( Reference Lahoz, Alonso and Ordovas 71 , Reference Ruiz-Gutierrez, Muriana and Guerrero 72 ). This beneficial effect is related to the high MUFA and polyphenol contents of EVOO( Reference Alonso, Ruiz-Gutierrez and Martinez-Gonzalez 73 ). The atherogenic index (total cholesterol:HDL) did not change significantly after 12 weeks of EVOO consumption, but it had a tendency to decrease in the elderly volunteers.
While EVOO consumption did not have a significant effect on the anti-inflammatory activities of Y-HDL and Y-PON1, this does not obviate the fact that it has a beneficial effect, especially in preventing CVD in both young and elderly populations. While elderly individuals, who are more subject to high oxidative stress and inflammation, may benefit the most from EVOO supplementation, an antioxidant- and polyphenol-rich diet could contribute to preventing the development of oxidative stress conditions and maintaining optimal cardioprotective functions, even in the absence of CVD risk factors.
In conclusion, the present results showed that there is a significant decrease in the anti-inflammatory activity of HDL with age and that PON1 is involved in the regulation of this atheroprotective activity. The decrease in activity was independent of plasma HDL concentrations and was probably due to the oxidative stress conditions that occur with ageing. An EVOO-rich diet could significantly reduce the age-related decreases in the anti-inflammatory activities of HDL and PON1 by reducing or preventing the damage caused by oxidative stress. The present results indicated that EVOO consumption increases the anti-inflammatory activity of HDL, which may explain its beneficial effect on CVD, and that an antioxidant-enriched diet is important, especially in elderly populations. Nevertheless, the present study has some limitations: (1) the design of the study lacks a control group or washout period before the EVOO intervention; (2) the diet of the participants was not controlled. Indeed, dietary changes, besides EVOO consumption, could promote an increase in HDL functionality (i.e. other polyphenols or antioxidants); (3) the sample is too small to allow firm conclusions to be drawn or to extrapolate the obtained results to a general population. Therefore, due to these limitations, the present study should be considered as a pilot study. However, further studies, considering these limitations, are needed to confirm the present results.
Acknowledgements
The present study was supported by a grant from the Canadian Institutes of Health Research (MOP-89912). The authors' contributions were as follows: A. K. designed the study and obtained funds from the Canadian Institutes of Health Research; S. L. carried out the experiments about the in vivo effect of olive oil; H. B. and O. H. participated in the sample collections and the preparation of the lipoproteins; S. I. and P. C. completed the experiments related to the revision of the manuscript; S. L. drafted the manuscript; A. K. revised the manuscript and completed the discussion. All authors read and approved the final manuscript. The authors declare that there are no conflicts of interest.













