INTRODUCTION
Based on available data from 10 European countries (collected between 2002 and 2006), the prevalence of current injecting drug users (IDUs) in the European Union has been estimated to be about 2·5 cases/1000 in the population aged 15–64 years, corresponding to between 750 000 and 1 000 000 individuals [Reference Wiessing1, 2]. IDUs are particularly vulnerable to bloodborne pathogens, such as hepatitis B virus (HBV), hepatitis C virus (HCV) and HIV, as a result of sharing contaminated syringes and other injecting equipment [Reference Friedland3]. Prevalence of HCV infection in IDUs in different parts of Europe varies from 18% to 98% [4–Reference Matheï, Buntinx and van Damme6] representing a significant burden of disease.
A range of harm reduction measures have been developed aimed at reducing the transmission of bloodborne pathogens in IDUs. These include the delivery of opioid substitution treatment, needle-exchange programmes, distribution of other injection-related paraphernalia, hepatitis B vaccination and drug consumption rooms [7–12]. Their existence and dates of implementation vary across Europe [4, Reference Hedrich, Pirona and Wiessing13]. Monitoring the prevalence of viral infections in IDUs within these countries and elsewhere is thus crucial to inform on the effectiveness of harm reduction measures to prevent the spread of infection. Studies aimed at estimating the prevalence of viral infections in IDUs face the challenge of capturing a relatively hidden and difficult to reach population, due to the illegal nature of injecting drug use. Choice of recruitment setting is therefore a key aspect of such study designs. Recruitment can be community-based, taking place in the street or drug scenes or service-based. The latest, logistically less demanding, is the most commonly reported. This type of recruitment setting traditionally includes services targeting drug users, such as drug-treatment centres and low-threshold services. While drug-treatment centres aim to reduce illicit drug use in clients, low-threshold services aim to reach individuals with problematical levels of drug use in order to prevent damage to their health, while not requesting abstinence from drug use [14]. Low-threshold services may include counselling, needle and syringe exchange programmes, shelter and medical care. Although populations are known to be different across settings, the impact of recruiting through either of these settings on prevalence estimation remains to be quantified.
Gaining insight into the influence of different recruitment strategies on HCV prevalence estimates will help inform surveillance activities across Europe. Therefore, we examined the association between recruitment setting and HCV prevalence in IDUs recruited from 12 surveys in nine countries across Europe.
METHODS
Data source
A multi-country collaborative project set up by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) brought together researchers across Europe (including some countries from outside the European Union) to share data from studies on IDUs in order to perform comparative analyses of bloodborne viral (BBV) infections in IDUs in Europe.
In November 2009, the members of this group were requested to provide individual-level survey data from IDUs containing information on HCV antibody status (from blood/saliva testing), recruitment setting and other demographic and behavioural data (see below).
Data collected
Twelve datasets originating from nine European countries were gathered [Reference Zabransky15–Reference Taylor22]. While datasets could include both IDUs and non-IDUs, the datasets used for this analysis were confined to ever IDUs. The coding of variables was harmonized across studies and datasets were merged.
Study-level information regarding study design, sampling strategy, geographical target population, criteria of inclusion and type of biological specimen sampled was collected through a questionnaire sent to the main investigators.
Outcome measure
The outcome measure was the result of the HCV antibodies test (i.e. positive/exposed or negative/unexposed) generated from either a blood or saliva specimen.
Study designs
Ten of 12 studies were BBV prevalence surveys, especially designed for that purpose. The two remaining studies originated from routine diagnostic testing performed in drug-treatment centres.
Recruitment setting
Recruitment setting data were provided by the data providers. They were then classified into three categories (drug treatment, low threshold, and other settings). The drug-treatment centres included inpatient and outpatient services providing detoxification and maintenance programmes to those attending these services. The low-threshold settings mainly included needle-exchange programmes and other outreach/harm reduction services. Finally, other settings included those recruited on the street, through custodies or specialized health services.
Statistical analysis
Univariate and multivariate analyses were performed in order to identify risk factors for HCV antibody positive status. Variables used in the analysis included recruitment setting, gender, HIV antibody test result, duration of injecting (in years), current injection status and frequency of injecting when available.
In univariate analysis, HCV antibody prevalence was compared to categorical variables using a χ2 test, with P < 0·05 indicating significance.
In order to estimate the adjusted influence of recruitment setting on the outcome of HCV antibody test, a multilevel analysis was performed. To account for variation between the studies, a mixed-effects logistic regression model was used. This model was built to determine the adjusted effect of recruitment setting on HCV outcome, accounting for variability between studies by entering the study identifier as a random intercept in the model. Other factors adjusted for in the model included gender, duration of injecting and current injection status (defined as injection in the last month).
In sensitivity analysis, we confined analysis and applied the model to studies which had undertaken recruitment at both drug-treatment and low-threshold settings. As those studies based on diagnostic testing recruited individuals at drug-treatment centres only, this sensitivity analysis was also thus confined to HCV prevalence surveys. All the statistical analyses were performed using Stata v. 10.0 software (StataCorp., USA).
RESULTS
Data originated from 12 studies and included between 196 and 1965 (median 625) individuals; a total of 8479 IDUs, recruited between 1990 and 2007, were used for this analysis (Table 1a). Inclusion criteria mainly relied on demographic traits and current injection status. The characteristics of the different studies included in the data analysis are presented in Tables 1a and 1b. Eleven of the 12 studies were conducted at sub-national or city level. The two diagnostic-based surveys followed an exhaustive sampling strategy including only IDUs recruited at treatment centres whereas the type of strategies used for seroprevalence surveys mainly included convenience sampling and snowballing or similar techniques. Moreover, half of these seroprevalence surveys included more than one type of recruitment setting. While six out of the 12 studies employed an age criterion to include individuals, the Swedish study excluded individuals who previously tested HIV positive and the Italian diagnostic testing-based survey included only heroin users. HIV prevalence varied from 1·1% to 25·6% across studies (Table 1b). In terms of sample demographics, individuals were the youngest and the mean duration of injecting was shortest in Czech Republic, Spain, Moldova and Poland. In contrast, the mean age was >40 years in Belgium, where IDUs had been injecting for the longest period of time. Finally, the proportion of female IDUs varied between 11% [The Netherlands (1)] and 35% [Czech Republic (1)].
Table 1a. Characteristics of the studies included in the analysis (restricted to the injecting drug users included in the studies), 1990–2007

Ab, Antibodies; DT, diagnostic testing, originating from data routinely collected during screening at the treatment centres; n.a., data not available; SP, seroprevalence study.
Table 1b. Characteristics of the studies included in the analysis, 1990–2007

IDUs, Injecting drug users; n.a., data not available.
HCV prevalence was 21–86% (overall prevalence 63%) across studies (Fig. 1). The prevalence point estimates were highest in IDUs included in drug-treatment centres. Indeed, half of the drug-treatment sites show a prevalence higher than 75% [Belgium, Italy (1, 2), Sweden]. In contrast, four of the six sites where IDUs were recruited in low-threshold services show prevalence below 45% and the highest prevalence estimate in this recruitment setting was 60% in Scotland. Prevalence estimates observed in other settings (including custodies and STI clinics) varied from 39% in The Netherlands to 88% in Sweden. Data was collected over an 18-year period. However, no trend in HCV prevalence could be detected over time.

Fig. 1. Hepatitis C prevalence (with 95% confidence intervals) by study and recruitment setting in 12 studies across Europe, 1990–2007 (ordered by date of data collection). Presentation of the types of recruitment settings used.
The overall HCV prevalence was significantly higher in IDUs recruited in drug-treatment centres compared to those recruited in low-threshold settings (74% and 42%, respectively, P < 0·001) (Table 2). Moreover, factors traditionally associated with HCV infection in IDUs were also found: significantly higher prevalence was found in males, those with longer duration of injecting, those HIV positive and those injecting more often.
Table 2. HCV prevalence by risk factors and study traits, in 12 studies across Europe, 1990–2007*
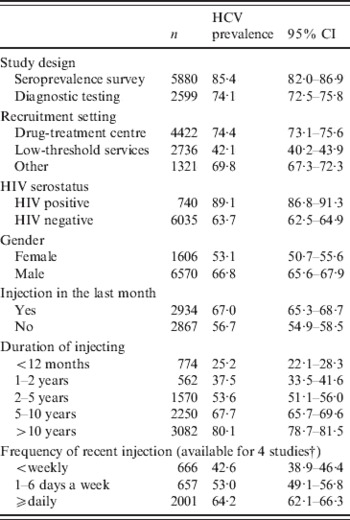
CI, Confidence interval.
* All association were statistically significant with P < 0·01.
† Frequency of recent injection available for studies: Czech Republic (1), Poland, Spain and Scotland.
Table 3 shows the characteristics of IDUs recruited in different recruitment settings. While HCV prevalence was significantly lower in IDUs recruited in low-threshold services (P < 0·001), HIV prevalence was significantly higher in this group compared to IDUs recruited in the drug-treatment centres (P < 0·001). Furthermore, the proportion of women and current injectors was significantly higher in those recruited from low-threshold services. Finally, the IDUs recruited from the drug-treatment centres had been injecting for a longer period of time and, in current injectors, individuals were injecting more often than those recruited from the low-threshold services (P < 0·001).
Table 3. Attendees' selected characteristics per recruitment setting, 12 studies across Europe, 1990–2007*
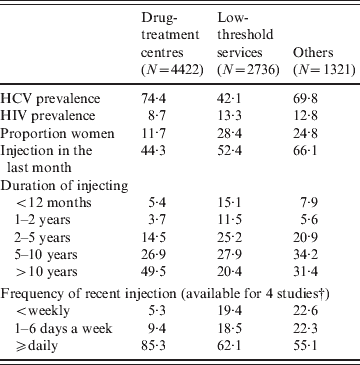
* All differences between drug treatment centres and low threshold services are significant (P < 0·001).
† Frequency of recent injection available for studies: Czech Republic (1), Poland, Spain and Scotland.
Recruitment setting remained significantly associated with HCV prevalence in IDUs after adjustment for duration of injecting and injection in the last month. The odds of being HCV positive was significantly lower in those recruited in low-threshold settings compared to drug-treatment centres [adjusted odds ratio (aOR) 0·7, 95% (CI) 0·6–0·8, P = 0·05] (Table 4a).
Table 4a. Multilevel analysis through hierarchical logistic regression. Factors associated with HCV seropositivity in 12 studies across Europe, 1990–2007 (N = 8479)

OR, Odds ratio; CI, confidence interval.
† P > 0·05. All other associations are significant with P < 0·001.
In order to test the robustness of the model, it was applied to the three countries in which more than one recruitment setting was used. While the association between recruitment setting and HCV status was not significant in Scotland (aOR 0·9, 95% CI 0·7–1·2, P = 0·501), it was significant in The Netherlands (2) study (aOR 0·4, 95% CI 0·3–0·7, P < 0·001) and borderline significant in Poland (aOR 0·7, 95% CI 0·5–1·0, P = 0·056) and always in the same direction as the findings displayed above. When the data from these three countries were pooled together, HCV prevalence was significantly higher in IDUs recruited in drug-treatment centres (Table 4b). When the two studies based on routine diagnostic testing (both based in drug-treatment centres) were excluded from the pooled analysis, the association remained significant (P < 0·01).
Table 4b. Multilevel analysis through hierarchical logistic regression. Factors associated with HCV seropositivity in studies including recruitment from both low-threshold and drug-treatment services [The Netherlands (2), Poland and Scotland], 1990–2007 (N = 2418)

OR, Odds ratio; CI, confidence interval.
† P > 0·05, * 0·001<P < 0·05. All other associations are significant with P < 0·001.
DISCUSSION
We studied factors associated with anti-HCV positivity in a pooled analysis of 12 European studies on IDUs. Our study highlights an association between the recruitment setting and HCV prevalence in IDUs, even after adjustment for key risk factors. This analysis is the first assessment of the association between recruitment setting and HCV on such a large sample size. It raises interesting questions and further research on this topic would be of added value.
Our analyses highlight differences in the characteristics of IDU populations recruited in low-threshold and drug-treatment services. As expected, more current injectors attended low-threshold services, for which there is no request for abstinence from drug use. Longer term injectors were more likely to be recruited in drug-treatment centres, which could be explained by the fact that drug users usually start treatment only after a certain period of time since starting drug use. The gender proportion was also different across settings, with females more likely to be found in low-threshold services. This observation suggests that female drug users may have less access to treatment or be less prompt or prepared to start this process as previously reported in the literature [Reference Greenfield23].
IDUs recruited in low-threshold settings had a lower HCV prevalence but a higher HIV prevalence compared to those recruited in drug-treatment centres. Previous studies found needle-exchange programmes as being associated with higher HIV prevalence [Reference Schechter24]. A recent study performed in Moscow suggested that new injectors are engaging in less risky injecting behaviours, but proportionally more reported exchanging sex in the last 4 weeks [Reference Platt25]. This finding could then partially explain our results as IDUs recruited in low-threshold settings have on average been injecting for a shorter period of time. Behavioural data covering sexual habits would have then been useful to further understand our results.
HIV and HCV main routes of transmission are different and therefore different patterns in their prevalence in distinct IDU populations could be expected. While HCV is not easily transmitted through sexual contact [Reference Alter26], bloodborne transmission of HCV has been proven to be much more efficient than that of HIV [Reference Gerberding27]. Data from the literature shows a significant decline in needle sharing in past decades [Reference Lindenburg28], while the sharing of other injection paraphernalia remains an issue for IDUs [Reference Hagan and Des Jarlais29]. These injection paraphernalia, including water for rinsing needles, cotton swabs for filtering drug solutions, and ‘cookers’ such as spoons or bottle caps for dissolving drugs, can be the transfer vector of small amounts of blood, potentially sufficient to ensure HCV but not HIV transmission [Reference Hagan30].
This analysis faces several limitations, which should be taken into consideration. First, the dataset includes data originating from 12 independent studies, with objectives differing from the one described in this paper. These studies were performed over 18 years, using different data collection tools and laboratory tests have evolved over time. We could not detect a trend in HCV prevalence over time. The evolution of the sensitivity of the laboratory tests should not impact on the association between recruitment setting and HCV outcome as no trend could be seen in the type of recruitment settings used. Inclusion criteria were also different across studies. While demographic criteria could be corrected for in the analysis, some behavioural criteria, such as main type of drug used, could have led to potential biases. Indeed, the impact of drug use on risk behaviour is highly dependent on the type of drugs involved. These limitations are the inevitable drawback of the use of a secondary combined dataset. Although we could adjust on duration and current status of injecting, the small number of behavioural variables has probably led to missing potential confounders that could have helped better understand our findings. Additional demographic information could bring a better insight on the type of individuals attending the different services. A more marginalized population may be expected to be found in the low-threshold services. Lifestyle is often associated with increased risky behaviour patterns.
A limited number of studies with more than one type of settings were included in the analysis, potentially weakening the multilevel adjustment used in this paper. In order to at least partly adjust for this limitation, we confined our analysis to studies having used more than one recruitment setting. Two of the three datasets generated from studies including more than one setting showed similar results as the overall ones. When pooled, data originating from these three studies showed a significantly higher HCV prevalence in drug-treatment centres.
The choice of a recruitment setting can rely on different decisional parameters including methodological preferences of the researchers, willingness of services to be included in the study, costs involved in recruiting in different settings or study objectives. Based on our findings, it is reasonable to recommend a mixed approach in terms of choice of recruitment setting in order to limit selection biases and ‘average out’ the impact of the various recruitment settings on the outcome. These findings are in line with recommendations formulated in the existing EMCDDA guidance [31]. Moreover, while including both low-threshold and drug-treatment centres can help in evaluating the impact of programmes on infectious disease prevalence, it limits the population captured to IDUs already in contact with the healthcare system. This source population might only be representing the visible part of the overall IDU population. Recruitment strategies aiming at achieving more representative samples of high-risk, difficult-to-reach populations have been developed in the last decades, including snowball sampling and respondent-driven sampling (RDS) [Reference Abdul-Quader32, Reference Heckathorn, Douglas33]. Snowball sampling involves individuals recruiting future subjects from among their acquaintances. RDS relies on snowball sampling and takes into account that study participants were not recruited randomly. It uses statistical weights based on the participants' network size and recruitment patterns to yield estimates of characteristics of the target population and the confidence intervals around the estimate [Reference Heckathorn, Douglas33]. The setting of these studies is usually the street or the drug use scene and they can bring new insights into the coverage of the healthcare systems targeting IDUs [Reference Malekinejad34]. The population captured by these studies can be expected to be a mix of IDUs attending drug-treatment centres, low-threshold services, none of them or both of them. Moreover, several reports have shown that RDS is an appropriate strategy to recruit IDUs [Reference Uusküla35]. A recent study aimed at comparing the characteristics of drug users recruited through RDS or target street outreach showed the samples recruited differed on many demographic and behavioural aspects [Reference Rudolph36]. Evaluation of the differences in HCV prevalence obtained from studies that had recruited IDUs attending services compared to through a RDS approach would be valuable to further guide drug-related infectious disease surveillance in Europe.
ACKNOWLEDGEMENTS
This study contributes to the work of the ‘European Study Group for Mathematical Modelling and Epidemiological Analysis of Drug-Related Infectious Diseases, coordinated by EMCDDA and RIVM with funding from WHO/Europe and the government of The Netherlands.
DECLARATION OF INTEREST
None.











