Article contents
Designing porous silicon-based microparticles as carriers for controlled delivery of mitoxantrone dihydrochloride
Published online by Cambridge University Press: 13 December 2012
Abstract
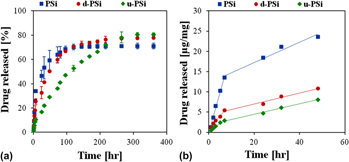
Porous silicon (PSi) microparticles are designed as tunable delivery carriers for the model anticancer drug, mitoxantrone dihydrochloride (MTX). The surface of the native nanostructured PSi particles was chemically modified by grafting dodecyl and undecanoic acid via thermal hydrosilylation with 1-dodecene and undecylenic acid, respectively. Attenuated total reflectance Fourier transform infrared spectroscopy and nitrogen adsorption–desorption measurements were used to characterize the physiochemical properties of the native and chemically modified PSi microparticles. MTX was loaded by physical adsorption to the native and dodecyl-terminated PSi or by covalent attachment to the undecanoic acid-terminated microparticles. Both drug release profile and the Si erosion of the carriers were significantly affected by the surface chemistry of the PSi microparticles and the drug loading method. The MTX release spans over a period of several hours to weeks, as dictated by these parameters. In vitro cytotoxicity studies on human breast carcinoma (MDA-MB-231) cells revealed that the released MTX maintains its cytotoxic functionality, in comparison to the very low toxicity of all PSi microparticles.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 2: Focus Section: Silicon-based Nanoparticles for Biosensing and Biomedical Applications , 28 January 2013 , pp. 231 - 239
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 16
- Cited by




