INTRODUCTION
Globally, the number of cholera cases reported to the World Health Organization (WHO) increased by approximately 16% between 2008 and 2009. This expanding burden of disease is heavily concentrated in sub-Saharan Africa, an area which accounted for 98% of cholera cases and 99% of cholera deaths reported to the WHO in 2009 [1]. In the East African nation of Uganda, cholera has become near-endemic, with cases documented each year from 2000 to 2009 [1, Reference Gaffga2]. While many parts of the country have long experienced repeated outbreaks of the disease, the Karamoja sub-region of northeastern Uganda has remained relatively unaffected. Prior to April 2010, when a cholera outbreak emerged in Karamoja, the region had not reported cases of the disease since 1997.
The Karamoja sub-region of Uganda is located in the country's northeastern corner and is composed of seven districts: Kotido, Nakapiripirit, Abim, Amudat, Napak, Kaabong, and Moroto. It is bordered to the north by Sudan and to the east by Kenya and has a population of roughly 1 million (Fig. 1). Historically, many of the region's inhabitants, primarily members of the loosely termed Karamojong ethnic group, derived livelihoods from nomadic pastoralist cattle herding. Over time this lifestyle largely shifted to one of ‘agro-pastoralism’ which combines nomadic pastoralism with agricultural cultivation and trade. With regards to settlement in Karamoja, patterns in much of the region have traditionally been twofold in nature. During the rainy season, most of the population resided in scattered villages, or manyattas, to facilitate cultivation and local cattle grazing. In the following dry season, many residents of the manyatta relocated with their cattle to mobile camps called kraals in search of optimal water sources and pastures for their animals [Reference Stites3].
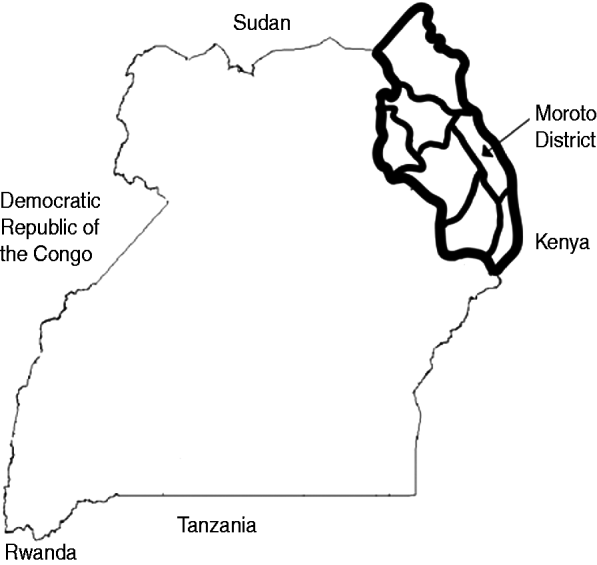
Fig. 1. Map of Uganda showing location of Karamoja sub-region and Moroto District. The heavy solid line (top right of Figure) represents the outline of the Karamoja sub-region. The medium-weight lines represent the seven districts comprising the sub-region.
Throughout its history, Karamoja has remained one of the poorest and marginalized areas of Uganda, in part because of several forms of chronic insecurity. Frequent periods of drought and famine have produced longstanding water and food insecurity, respectively. Decades of armed conflict due to internal and cross-border cattle raiding have resulted in a setting of civil insecurity. In recent years, these forms of insecurity, armed violence in particular, have resulted in a significant reduction in the traditional manyatta/kraal settlement pattern in Karamoja. In attempts to reduce conflict in the region, the government of Uganda has instituted disarmament campaigns and encouraged sedentism in place of mobility [Reference Stites and Akabwai4]. While mobile herding continues in much of the region, a growing percentage of the population has settled and now resides predominantly in less scattered and overcrowded manyattas, many of which lack adequate latrine coverage and access to safe water sources. Semi-permanent kraals have been established throughout the region, usually in the vicinity of Ugandan military barracks with the intent of providing protection from cattle raiders.
Between April and July 2010, an outbreak of cholera occurred in Moroto District, the regional capital of Karamoja. This was the first outbreak of the disease in the area since 1997. As a result of the extended time period between outbreaks, information regarding epidemiological trends and location-specific risk factors was not readily available. Furthermore, while a previous study had examined the effects of sedentism on the general health of nomadic pastoralist children in Kenya, no published data was available concerning the epidemiology of diarrhoeal disease and cholera among nomadic or semi-nomadic pastoralists in sub-Saharan Africa [Reference Nathan, Fratkin and Roth5]. We therefore conducted a case-control investigation during the outbreak to describe the epidemiology and identify risk factors for cholera transmission in a setting of semi-nomadic pastoralism. As nomadic pastoralists in sub-Saharan Africa continue to settle, the results of our study may be useful to public health authorities and cholera prevention and control programmes in similar environments.
METHODS
Ethics
The investigation was approved by the Committee on Research Involving Human Subjects at Albany Medical College and the Uganda National Council for Science and Technology. Written informed consent was obtained from all investigation participants or from their parents or guardians.
Descriptive epidemiology
Data collection
The comprehensive line list kept by the Moroto District Health Office was reviewed to determine all reported cholera cases in the district from April to July 2010. Information on demographics (gender, age, residence), date of illness onset, and date of hospital admission were abstracted in order to characterize the distribution and timeline of the outbreak. For the 26 cases that were missing information on date of illness onset, the median time lag between date of onset and hospital admission for the remainder of cases was calculated and applied after rounding to the nearest whole day. The population structure and projections by sub-county for Moroto District were obtained from the most recent Uganda Bureau of Statistics (UBOS) population and housing census data. Attack rates were computed by using the district population projections.
Case definition
Throughout the outbreak, in accordance with Uganda Ministry of Health (UMOH) surveillance guidelines, a cholera case was defined clinically as an individual of any age that: (1) had acute watery diarrhoea and; (2) was admitted to one of three cholera treatment centres (CTCs) established in Moroto. While the UMOH index case definition excludes those aged <5 years and includes onset of severe dehydration or death from watery diarrhoea, the broader definition described here was used following index confirmation of Vibrio cholerae O1 in Moroto to provide increased sensitivity and thorough case detection.
Microbiological analysis
Examination of stool samples from the index cluster of patients was performed at the UMOH's Central Public Health Laboratories (CPHL) in Kampala. Following collection in Moroto, the samples were transported to CPHL in Cary–Blair media and plated on thiosulfate-citrate-bile salts-sucrose (TCBS) agar for microbiological analyses. Once identified, V. cholerae isolates were further evaluated to determine the corresponding serogroup and serotype. To guide the clinical management of severe cases with antimicrobials, sensitivity testing was performed to determine the profile of resistance in the identified samples of V. cholerae.
Case-control investigation
In order to determine potential risk factors and prevention and control opportunities during the outbreak, a case-control investigation was conducted in June 2010. An unmatched design was implemented due to logistical challenges presented by the nomadic lifestyle still followed by many inhabitants of the region of study. As nomadic mobility resulted in potential study subjects being geographically scattered with their animals by considerable distances for inconsistent periods of time, matching subjects was not logistically feasible.
Participants
Since the majority of cases at the time of study, as well as the index cluster, had originated from one sub-county in the district (Nadunget), we chose to focus our investigation on this sub-county's most affected villages. To specifically identify cases, we examined the demographic information of patients admitted to the CTCs established in Moroto. We then selected those patients residing in the 15 most affected villages in Nadunget sub-county, defined as the 15 villages that had reported the greatest number of admissions to the CTCs from April 2010 up until the time of investigation. Cases were defined as individuals who: (1) met the UMOH's outbreak case definition (acute watery diarrhoea in an area with laboratory-confirmed cholera cases); (2) were admitted to a CTC in Moroto during April–June 2010; and (3) resided in one of the 15 selected villages in Nadunget. Controls were defined as individuals that: (1) had not experienced any form of diarrhoea from April 2010 to the time of investigation; (2) resided in one of the 15 selected villages in Nadunget. Individuals aged <10 years were excluded from cases and controls due to difficulties in obtaining accurate information regarding personal behaviours and sanitation and hygiene practices.
Sample size
The sample size was determined using the StatCalc program from the Epi Info™ software package version 3.2.2 [Centers for Disease Control and Prevention, USA (http://www.cdc.gov/EpiInfo/)]. We based our calculation on findings from a study which found that 58% of cholera cases exhibited a previously reported risk factor for the disease (consumption of uncooked food), compared to 37% of controls [Reference Lucas6]. Therefore in order to identify risk factors for cholera in Moroto, with a 95% confidence interval; power of 80%; with a case/control ratio of 1 and looking for an odds ratio of 2 between cases and controls, it was determined that at least 97 cases and 97 controls would be adequate to determine risk factors. In total, data from 99 cases and 99 controls were analysed.
Data collection
A standardized questionnaire was developed with input from health inspectors and clinicians in Moroto who had knowledge of local practices that were suspected modes of transmission during the outbreak. The finalized questionnaire included sections on demographic information, travel history, funeral attendance, hygiene and sanitation practices, and eating and drinking habits from April 2010 to the interview date. The questionnaire was administered to cases and controls by trained local health workers recruited from across the district. The health workers visited cases and controls in their households to administer the questionnaire.
Statistical methods
Data were entered and cleaned using Epi Info software version 3.2.2. Errors in data entry were monitored by rechecking all electronic data with the original data collection forms. Following cleaning, bivariate and multivariate analyses were conducted in SPSS version 16.0 [SPSS Inc., USA (www.spss.com)].
Odds ratios (ORs), 95% confidence intervals (CI), and P values were calculated for the bivariate analysis. To control for confounding and to test for effect modification, we conducted a multivariate analysis. The bivariate phase served as a screening tool for variables to be included in the multivariate model. Variables were included in the initial multivariate model if their corresponding P value in the bivariate analysis was ⩽0·10. Analysis of the multivariate model was done using a binary logistic regression. Using a backwards elimination procedure, variables with the lowest statistical significance were progressively dropped from the model until only those with P<0·05 remained. To identify potential effect modification by age and gender, corresponding interaction terms were included in the final model. Interaction terms were considered significant if P<0·05.
RESULTS
Index case investigation and response
The index case was a 21-year-old female from Kanakomol village, Naitakwae parish, Nadunget sub-county in Moroto District. Her illness began on 21 April 2010 and she was admitted to Moroto Regional Referral Hospital on this date with profuse watery diarrhoea and vomiting suggestive of cholera. A second case presented to Moroto Hospital later on 21 April followed by a third case on 22 April, both resided in Kanakomol village.
The Moroto District rapid response team visited Kanakomol and observed that the borehole supplying water to the village was non-functional and the residents were using the Nadunget River as their main source of water for domestic use and consumption. The residents indicated to the rapid response team that drinking water from open sources was never chlorinated or boiled. Due to the complete absence of latrines in Kanakomol for a population of approximately 670 persons, residents were observed by the rapid response team using the Nadunget River for defecation and the team observed human faeces on the river bed. Subsequent testing of Nadunget River water revealed heavy contamination with faecal coliforms. It was therefore suspected that the outbreak originated from drinking contaminated water from the Nadunget River. In response to these observations and findings, a water tank was provided to Kanakomol village and was continually refilled by a water tanker. Kanakomol residents were mobilized to dig and use pit latrines for faecal disposal. Approximately 50% of cases reported during the first week of the outbreak resided in Kanakomol. Subsequent cases were reported elsewhere in Moroto District with most of the affected villages located along the Nadunget River.
Microbiological findings
Stool samples tested at CPHL collected from the first four cases were uniformly positive for V. cholerae serogroup O1, serotype Inaba. Antimicrobial sensitivity testing found all samples completely resistant to chloramphenicol, nalidixic acid, and cotrimoxazole and intermediately resistant to ampicillin. All samples were sensitive to tetracycline and ciprofloxacin. No other pathogens were isolated from stool.
Village health teams (VHTs)
In response to the outbreak, the UMOH and its partners increased support for community-based VHTs. VHTs are composed of members of the local community who are trained to recognize and preliminarily treat basic illnesses and to facilitate health education and promotional activities in their villages. As many residents of Moroto District followed nomadic lifestyles and often resided in manyattas far from towns and trading centres, VHTs were utilized to execute a variety of cholera surveillance, prevention, control, and treatment activities in these remote and relatively inaccessible communities. These included case recognition and notification, digging of pit latrines, chlorine tablet and oral rehydration solution (ORS) distribution, and cholera-specific health education activities.
Case management
During the outbreak, ORS was made available in the affected villages by supplying them to VHTs as well as the local drug shops. All patients with acute watery diarrhoea were encouraged to start on treatment with ORS and report immediately to the nearest CTC. Ambulance services based at Moroto Hospital were made available to transport cases from their residences to the CTCs. Healthcare workers at each CTC were trained on infection control procedures and on the management of cholera patients based on a standard treatment protocol. For all patients admitted to a CTC, an assessment for the level of dehydration was made by a trained healthcare worker. All patients with no dehydration or mild dehydration were managed on oral rehydration therapy using ORS. All severely dehydrated patients were managed on intravenous Lactated Ringer's solution together with ciprofloxacin in accordance with the results of antimicrobial sensitivity testing. Feeding was encouraged for all patients. Patients were discharged from the CTC after all vomiting and diarrhoea had subsided.
Descriptive epidemiology
In total, 641 cholera cases were identified in Moroto between 21 April and 14 July 2010, when the last case was admitted to the CTC at Moroto Hospital. The median age of cases identified during the outbreak was 19 years (range 0·5–81 years). Fifty-five percent were female. For cases with both dates available (615 cases), the median time lag between date of illness onset and date of hospital admission was 1 day. Most cases occurred between mid-May and early June 2010 with the largest number of cases occurring with onset between 16 and 30 May 2010 (Fig. 2). Ten patients died, mostly in the community, at the onset of the outbreak (case-fatality rate 1·56%). Of the cases that died, it was determined that most did not recognize the severity of their illness and did not seek medical attention or rehydration therapy. Other patients died because they presented to treatment centres at a late stage in their illness and did not respond to rehydration and volume replacement therapy.
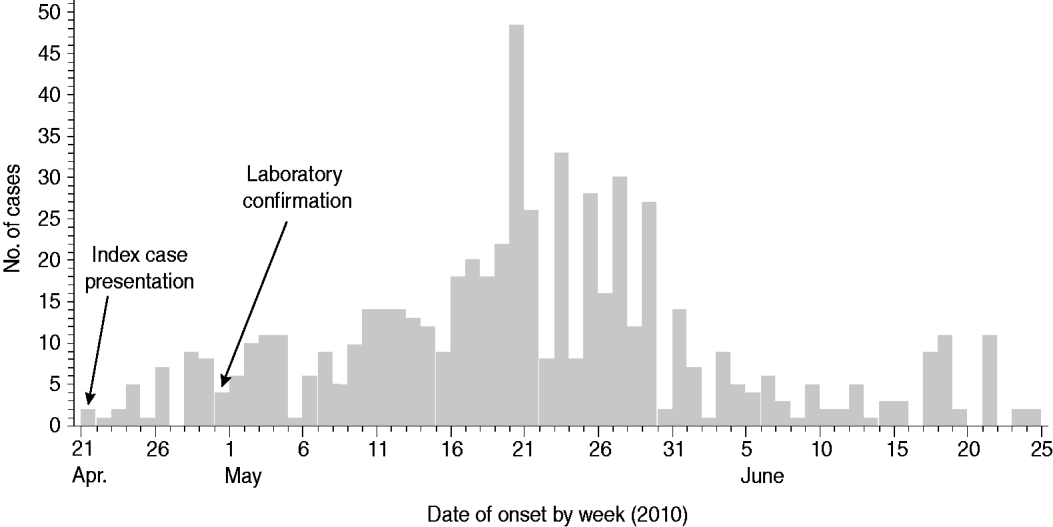
Fig. 2. Epidemiological curve for the cholera outbreak in Moroto District, Uganda, April–July 2010. ‘Index case presentation’ indicates the date that the first two cases presented to Moroto Hospital. ‘Laboratory confirmation’ indicates the date of isolation of Vibrio cholerae O1 by the Uganda Ministry of Health's Central Public Health Laboratories. Day-specific listings were unavailable for cases with illness onset from 1 to 14 July 2010.
The overall attack rate for Moroto District was 21·5/10 000 persons. While cholera cases were reported from all 11 sub-counties in the district, most cases were found in Nadunget (46·9%), Rupa (26·8%), South Division (14·5%), and North Division (5·5%). South Division represented the highest attack rate (153·8/10 000) followed by Nadunget (70·9/10 000) (Table 1). When attack rates were calculated according to age groups, the 0–9 years age group was the highest (39·3/10 000) followed by the 30–39 years group (25·0/10 000) (Table 2).
Table 1. Attack rates by sub-county for the cholera outbreak in Moroto District
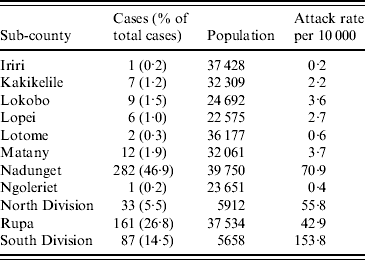
Data unavailable for 40 cases.
Table 2. Attack rates by age group for the cholera outbreak in Moroto District
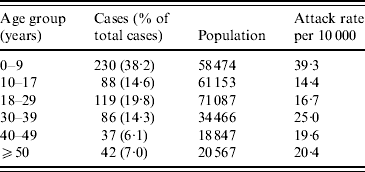
Data unavailable for 39 cases.
Case-control investigation
Data collected from 99 cases and 99 controls were analysed to determine risk factors that might indicate modes of cholera transmission during the outbreak. The median age of cases was 26 years, with the 10–17 and 18–29 years age groups leading the distribution almost equally. Most cases were female (64·6%). Crop farming represented the most common occupation of cases (37·4%). For controls, the median age was 33 years, with the 18–29 years age group leading the distribution. Regarding gender for controls, there were slightly more females (51·5%) than males. The most common occupation of controls was that of labourer (31·3%). Cases and controls were similar in distribution by education level (Table 3).
Table 3. Demographic characteristics of case, control, and total subjects enrolled in case-control investigation
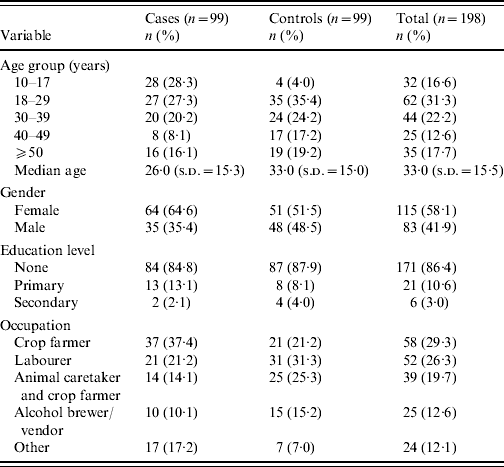
s.d., Standard deviation.
In bivariate analysis, 11 risk factors were found to be significantly associated with symptomatic cholera at P<0·05 (Table 4). In terms of sanitation and hygiene practices, cases were less likely than controls to have a latrine present in their household (OR 4·8, 95% CI 1·3–17·5) and less likely than controls to dispose of children's faeces in a latrine (OR 13·5, 95% CI 1·7–106·1). Not washing hands following defecation was also associated with an increased risk of symptomatic cholera (OR 11·0, 95% CI 4·4–27·4).
Table 4. Results of bivariate and multivariate analyses to assess potential risk factors
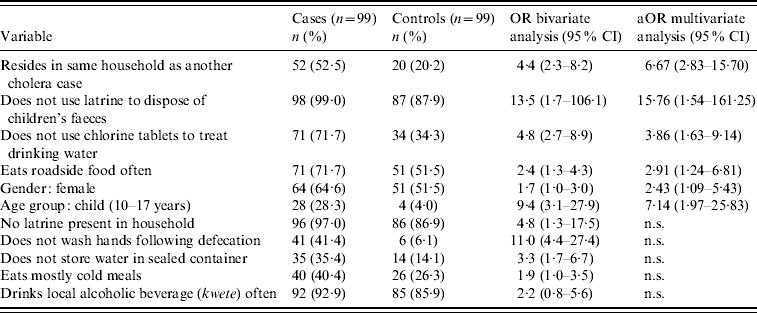
OR, Odds ratio; aOR, adjusted odds ratio; CI, confidence interval; n.s., variables not significant at P<0·05 in multivariate analysis.
Several eating and drinking habits were found to be significant risk factors in the bivariate analysis. Cases were more likely than controls to eat cold meals (OR 1·9, 95% CI 1·0–3·5) and roadside-vended food (OR 2·4, 95% CI 1·3–4·3). Drinking unchlorinated water represented increased risk of disease (OR 4·8, 95% CI 2·7–8·9) as did not storing drinking water in a sealed container (OR 3·3, 95% CI 1·7–6·7).
Demographically, cases were more likely than controls to be children, defined as those aged 10–17 years (OR 9·4, 95% CI 3·1–27·9), and to have a member of the same household diagnosed with cholera (OR 4·4, 95% CI 2·3–8·2).
While they did not reach statistical significance at P<0·05 in the bivariate analysis, female gender and consumption of a locally brewed alcoholic beverage (kwete) appeared to be associated with increased risk and were also included in the multivariate model (Table 4).
In multivariate binary logistic regression models, six risk factors remained significantly associated with symptomatic cholera at P<0·05 (Table 4). Having another member of the household diagnosed with cholera remained significantly associated with disease [adjusted OR (aOR) 6·67, 95% CI 2·83–15·70] as did failing to dispose of children's faeces in a latrine (aOR 15·76, 95% CI 1·54–161·25). Compared to controls, cases remained more likely to drink unchlorinated water (aOR 3·86, 95% CI 1·63–9·14) and to eat roadside food (aOR 2·91, 95% CI 1·24–6·81). Cases were more likely than controls to be female (aOR 2·43, 95% CI 1·09–5·43) and to be a child (aOR 7·14, 95% CI 1·97–25·83). No significant effect modification by age or gender was observed.
DISCUSSION
Due to long-term water, food, and civil insecurity, many nomadic pastoralists in sub-Saharan Africa, including those in the Karamoja region of Uganda, have begun to experience changes in livelihood strategies and increased patterns of sedentism. This more settled and semi-nomadic way of life presents challenges for public health authorities. Certain behaviours inherited from nomadic and mobile lifestyles may become risk factors for cholera in a sedentary and overcrowded environment. Our investigation is the first to report on the epidemiology of and risk factors for cholera in this setting.
Compared to controls, cases were more likely to reside in the same household as another person diagnosed with cholera. Studies from India and Bangladesh have demonstrated similar epidemiology for symptomatic infection with V. cholerae [Reference Sur7, Reference Weil8]. Factors that are likely to contribute to increased intra-household risk of V. cholerae in Moroto (which has an average household size of 5·1 persons according to the most recent UBOS data) include the increasing population density in many of the manyattas and poor sanitation. Together, these factors create an environment conducive to the faecal–oral transmission of V. cholerae. Apparent person-to-person transmission may occur through household water and food. These are more likely to be contaminated if a cholera case resides in the home, which is common since sick persons are frequently cared for in the home at the onset of illness. Persons residing in the same household as a cholera case may therefore need to be particularly targeted with health education campaigns. During the outbreak in Moroto, household members who accompanied their relatives to CTCs were specially educated on cholera prevention measures and on the need to mobilize their communities.
It has been demonstrated in previous studies that treating drinking water with a chlorine-based disinfectant is effective in preventing cholera transmission [Reference Sasaki9–Reference Tauxe, Mintz and Quick11]. In our investigation, consumption of untreated water was significantly associated with increased risk of disease. Due to chronic water insecurity in Karamoja and common to nomadic lifestyles, water is collected and often directly consumed from open sources such as ponds, rivers, and puddles as soon as it becomes available following rains. These unprotected water sources are also common sites for bathing and open defecation and may therefore be contaminated with faecal matter.
The outbreak described here occurred during Karamoja's rainy season. As a result, there were an increased number of open sources available in the region for water consumption and simultaneous bathing and defecation. While boreholes usually contain more potable water than open sources, the coverage of functioning boreholes in Moroto at the initiation of the outbreak was only 42%. Water testing conducted by the Italian Development Cooperation during the outbreak also indicated the presence of faecal coliforms in three out of 14 randomly selected boreholes in the district. Considering the high risk posed by ingestion of water from unprotected or contaminated sources, strong attempts should be made to emphasize the importance of water chlorination as it represents a simple and effective way to avoid infection and illness. During the outbreak, VHTs encouraged chlorination of household water in their villages by distributing chlorine stock solution and chlorine tablets provided by UNICEF. Since members of the community at times resisted the use of chlorination products in drinking water due to taste complaints and a preference for water from open sources, VHTs carried out promotional campaigns emphasizing the risks of consuming water from contaminated sources and the need for disinfection.
Consumption of roadside-vended food was found to be a significant risk factor for cholera in Moroto, a finding similar to that reported from other outbreaks [Reference Lim-Quizon12, Reference Estrada-Garcia and Mintz13]. Drought and poor crop harvests have left many residents of Moroto with chronic food shortages and food insecurity [Reference Stites3, Reference Stites and Akabwai4]. The shortage of cultivated food leads residents to increase their consumption of roadside-vended food. Many of the food items sold at roadside sites are not served hot and there is often inadequate hand washing by vendors which may lead to increased contamination of food with V. cholerae. To address this issue, a ban on roadside food vending was instituted and health education on food hygiene was provided to food handlers in restaurants. A ban on the brewing and consumption of the local alcoholic beverage (kwete) was also instituted, but its enforcement was not as effective as that placed on roadside food vending.
Females were found to demonstrate a greater risk for symptomatic cholera than males. This result is similar to a finding from a study in Bangladesh which found a higher attack rate of cholera in young women compared to similarly aged men [Reference Glass14]. In Karamoja, women and girls traditionally remain in the manyatta to pursue domestic work while men and boys are more likely to travel with their animals to and from the kraals and to other locations for trade or labour. In addition to spending most of their time in the overcrowded manyattas, women are traditionally responsible for caring for the sick and this role may place them at greater risk for infection.
Sedentism among former nomadic pastoralists has been associated with increased incidence of diarrhoeal disease, particularly in young children. Among Rendille pastoralists from northern Kenya, children from sedentary communities had a higher incidence of diarrhoeal disease compared to those living nomadic lifestyles [Reference Nathan, Fratkin and Roth5]. While we did not directly compare risk between sedentary and nomadic communities, our results are consistent with these findings. Cholera cases in our increasingly sedentary, semi-nomadic sample were more likely to be children than controls. In addition, when attack rates were calculated, the 0–9 years age group led the distribution. Both of these findings suggest that children in Moroto faced an especially high-risk environment for cholera transmission. Young girls often remain in the manyattas to assist in domestic work while boys attend to cattle in the kraals and move with their animals to find locations to graze. Children may be exposed to V. cholerae in and around the kraals or manyattas through contact with contaminated faecal matter or direct consumption of water from unprotected sources.
There also existed a belief in Moroto that children's faeces were not infectious and did not need to be disposed of. In fact, cholera cases in our sample were less likely to dispose of children's faeces in a latrine compared to controls. In a 1995 study from India, it was found that an absence of latrines for disposal of faecal matter was a risk factor for cholera [Reference Singh15]. Considering the faecal–oral transmission of V. cholerae, those handling children's faeces may have come into contact with infectious organisms.
The most common occupations of cases and controls were crop farmers and labourers, respectively. This difference in occupation status of cases and controls may have been the result of the geographical location of the activities performed by each group of workers. Labourers often spend time in trading centres and large towns while performing their work. Crop farmers tend to remain in close proximity to their more rural homesteads. As a result, labourers may have received more exposure to health education and prevention activities, as well as sanitation supplies, at the onset of the outbreak before VHT and outreach programmes were fully executed in rural communities.
In Karamoja, the utilization of VHTs provided an important method of implementing cholera surveillance, prevention, control, and early treatment activities in remote and traditionally isolated nomadic and semi-nomadic pastoralist populations. Community-based involvement in outbreak response is critical in regions often troubled by years of civil conflict and in populations historically suspicious of outsiders or government involvement. As they are composed of members of the local community and are sensitive to the unique dynamics of nomadic pastoralist lifestyles, the utilization of VHTs represents an opportunity to make tailored improvements in the health of such populations in sub-Saharan Africa. During the outbreak, VHTs provided a highly sensitive surveillance network in their villages as they were able to quickly recognize and respond to suspected cholera cases among their peers, report them to the nearest CTC, and deliver early treatment with ORS while arranging transport. VHTs also educated their fellow community members on routes of cholera transmission unique to a nomadic pastoralist way of life. For example, recognizing that many members of their community resisted water chlorination, VHTs emphasized the risks posed by consumption of water from open sources while distributing chlorine tablets. VHTs also led the digging of pit latrines and highlighted their importance in waste disposal in order to combat open defecation. In leading by example among their peers, VHTs mobilized their unique communities to change behaviours closely linked to nomadic lifestyles. While further research is needed to explore and quantify their effectiveness, the outbreak response in Karamoja suggests that VHT programmes present a strong public health intervention for nomadic and semi-nomadic pastoralists transitioning into a high-risk environment for cholera transmission.
Our investigation had limitations. Methodologically, the investigation was limited by lifestyle factors intrinsic to the population affected by the outbreak. Matching subjects was not feasible because of the scattered geographical distribution and varied time schedules of the population. This unmatched design may have introduced bias into the enrolment of study participants. Second, because we conducted our investigation roughly 1 month after cholera cases were initially confirmed, there existed the potential for recall bias. Third, we chose to interview cases and controls from villages in Nadunget sub-county in Moroto as this was the sub-county that had reported the large majority of cases up until the time of investigation and was the home of the index cluster. While there may be behaviours or practices uniquely present or absent in Nadunget, we are confident that our chosen location represents the majority of the area affected by the outbreak. Finally, considering that most infections with V. cholerae O1 present asymptomatically or with only mild illness, controls in our sample may have been unknowingly infected. As this would weaken the strength of the observed risk associations, we follow the example of a previous investigation and suggest that our reported risk factors be viewed as those for symptomatic, or clinically significant cholera [Reference Shultz16].
The transition to sedentism among nomadic pastoralists presents unique challenges for public health authorities when addressing sanitation and hygiene-related diseases such as cholera. Civil insecurity has accelerated settlement in Karamoja in addition to weakening sanitation infrastructure in the region. When introduced into overcrowded manyattas, behaviours common to previously nomadic lifestyles such as open defecation and drinking of untreated water present risks for cholera and diarrhoeal disease. Long-term water and food insecurity may compound the problem by leading to other unsafe drinking and eating practices. Utilizing VHTs and their local knowledge to target these specific behaviours may allow for effective prevention when coupled with improvements in sanitation and accessibility to safe water sources. Further investigations are still needed to verify risk factors for cholera and other diarrhoeal diseases under high-risk circumstances similar to those observed in the Karamoja region of Uganda.
ACKNOWLEDGEMENTS
This study was supported by the David R. Nalin '65 Fund for International Research at Albany Medical College. We thank the staff of the Moroto District Health Office for their assistance in data collection.
DECLARATION OF INTEREST
None.










