Introduction
Migration is an adaptive behavioural trait that allows individual organisms to capitalize on resources that fluctuate in time and space. Despite energetically costly movements, selective forces increase individual fitness when resource scarcity or crowding by conspecifics makes habitats more risky or less attractive (Dingle & Drake, Reference Dingle and Drake2007). Migration conjures up images of thousands of wildebeest Connochaetes taurinus thundering across the Serengeti (Boone et al., Reference Boone, Thirgood and Hopcraft2008) or pods of humpback whales Megaptera novaeangliae on their annual journey from Antarctica to Central America (Rasmussen et al., Reference Rasmussen, Palacios, Calambokidis, Saborío, Dalla Rosa and Secchi2007). However, migratory pathways in prairie ecosystems that sustain endemic North American fauna are poorly conserved but equally important to global biodiversity. The loss of long-distance movements in North America’s sole surviving endemic ungulate, the pronghorn Antilocapra americana, typify the inability of small prairie reserves to sustain migratory populations (Berger, Reference Berger2004). Prairie conservation supports sedentary populations within a patchwork of fragments that, at times, results in inadvertent loss of stepping stones necessary to maintain migratory species. Maintaining connectivity in large and intact grasslands should be a primary conservation objective before opportunities to do so are lost.
Greater sage-grouse Centrocercus urophasianus (hereafter sage-grouse), categorized as Near Threatened on the IUCN Red List (BirdLife International, 2008), are representative of the struggle to maintain biodiversity in a landscape that bears the debt of ever-increasing demands for natural resources (Knick et al., Reference Knick, Dobkin, Rotenberry, Schroeder, Vander Haegen and van Riper2003). Expansion of the human footprint (Leu et al., Reference Leu, Hanser and Knick2008) continues to fragment the once vast tracts of sagebrush (Artemisia spp.)-dominated grasslands that sage-grouse require for each stage of their life-history (Connelly et al., Reference Connelly, Schroeder, Sands and Braun2000). This association is strongest in winter when sage-grouse forage in large dense stands of sagebrush that remain above snow (Homer et al., Reference Homer, Edwards, Ramsey and Price1993; Doherty et al., Reference Doherty, Naugle, Walker and Graham2008). This behaviour accounts for a diet of > 94% sagebrush in winter (Remington & Braun, Reference Remington and Braun1985) and results in high survival and weight gain for sage-grouse (Beck & Braun, Reference Beck and Braun1978). Sage-grouse conservation has largely focused on protecting nesting and brood-rearing habitats adjacent to leks because tracking research has largely focused on habitat around nest sites and brood-use locations (Connelly et al., Reference Connelly, Schroeder, Sands and Braun2000; Hagen et al., Reference Hagen, Connelly and Schroeder2007). However, recent findings show severe winter weather can decrease survival (Moynahan et al., Reference Moynahan, Lindberg and Thomas2006) and that human disturbance degrades otherwise suitable winter habitat (Doherty et al., Reference Doherty, Naugle, Walker and Graham2008; Carpenter et al., Reference Carpenter, Aldridge and Boyce2010).
Divergent migratory strategies across the range of sage-grouse reflect the variation in distribution and abundance of available habitats. Non-migratory populations fulfil annual habitat requirements within overlapping seasonal ranges, while populations that are obligate or partial migrants occupy spatially distinct breeding, summer or winter ranges (Beck et al., Reference Beck, Reese, Connelly and Lucia2006). Conservation plans often delineate bird concentration areas but lack the information on movement necessary to identify areas that support entire populations. One population in south-east Idaho travels c. 80 km between summer and winter (Connelly et al., Reference Connelly, Browers and Gates1988; Leonard et al., Reference Leonard, Reese and Connelly2000) and individuals from a nearby population move < 5 km between autumn and winter (Beck et al., Reference Beck, Reese, Connelly and Lucia2006). Identifying migratory pathways provides the biological basis for maintaining connectivity and conveys to decision makers the size of prairie landscapes necessary to support migratory populations.
We report the discovery of the longest documented migration ever recorded for any prairie grouse species. We found that sage-grouse in this population nested and raised broods in plains silver sagebrush Artemisia cana cana habitats but were surprised when birds moved up to 122 km south to winter in Wyoming big sagebrush habitats Artemisia tridentata wyomingensis. Birds probably migrated from sparse silver sagebrush to tall dense stands of big sagebrush in search of a more reliable food source in winter. This study includes individuals captured in the largest remaining active lek (communal breeding ground) in Canada. Our objectives are to (1) demonstrate that the fate of sage-grouse in Canada partly depends upon conservation efforts in the USA, (2) identify human stressors that could sever connectivity between countries, and (3) pinpoint the northern Great Plains as a high conservation priority internationally.
Study area
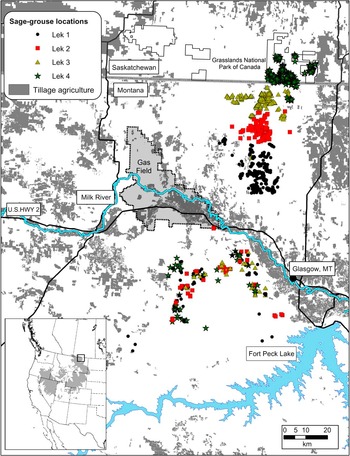
Our study area covered portions of Phillips and Valley counties in Montana, USA, and south-central Saskatchewan, Canada (Fig. 1). North of the Milk River is a short grass prairie ecosystem with a predominately native understorey of western Agropyron smithii and northern Agropyron dasytachyum wheatgrass communities. Plains silver sagebrush occurs in dense patches along linear overflow areas on the banks of seasonal streams and in sparse clumps in upland grasslands. A similar grassland understorey is found south of the Milk River but with a dominant shrub cover of Wyoming big sagebrush. Big sagebrush is a denser, more ubiquitous shrub than silver sagebrush, with large tracts (> 100 ha) occurring in uplands. Mean minimum temperatures in winter are -6.3°C, with a mean snowfall of 400 mm.
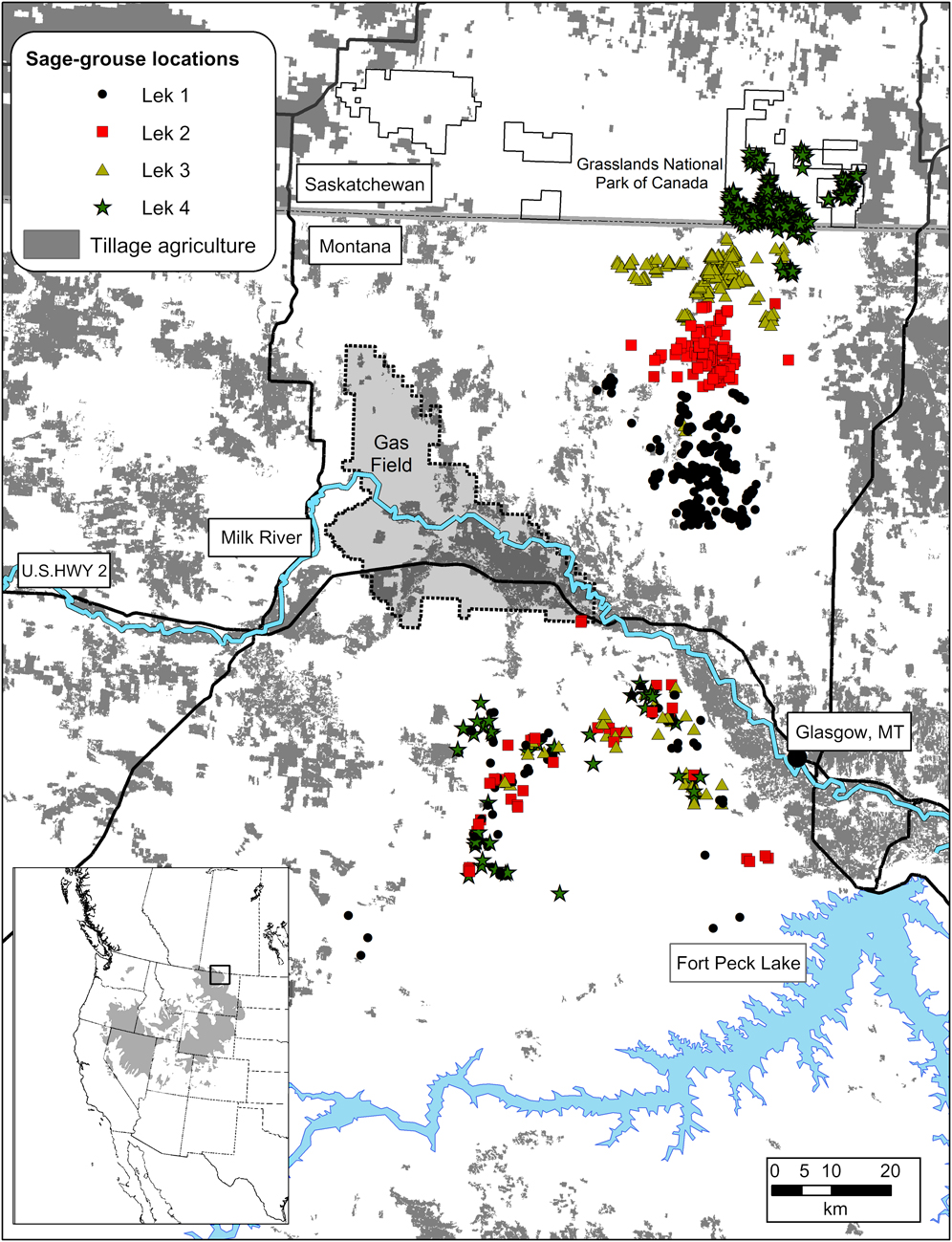
Fig. 1 Female greater sage-grouse Centrocercus urophasianus locations in spring and summer (north of Milk River) and winter (south of Milk River) in north-eastern Montana and the East Block of Grasslands National Park, Saskatchewan. The rectangle on the inset depicts the current occupied range of the species (Schroeder et al., Reference Schroeder, Aldridge, Apa, Bohne, Braun and Bunnell2004).
Methods
We captured 80 female sage-grouse on leks in northern Valley County, Montana, and in the East Block of Grasslands National Park, Saskatchewan, during the spring breeding seasons of 2007 and 2008 (Fig. 1). Sage-grouse were classified as yearlings or adults based on primary feather development (Eng, Reference Eng1963). Females were fitted with a 22-g necklace-style radio collar with an 18-h mortality switch (Advanced Telemetry Systems, Isanti, USA). Females were then banded with a size 20, individually-numbered, aluminium band (National Band and Tag Co., Newport, USA). We intensively followed marked birds from March to September, and conducted six flights each winter between November and March in 2008 and 2009 to relocate radio-marked females from a fixed-wing aircraft at 300–600 m above ground level (AGL). We circled marked birds at 30–100 m AGL until we reached maximum signal strength, and recorded their location with a global positioning system (GPS). An independent source placed 10 collars within the study area near known winter locations in habitat similar in vegetation and ruggedness to estimate location error. We calculated the distance between recorded and known locations of the training collars and used the maximum value (105 m) as our resolution to estimate locations.
This population used overlapping ranges during breeding and summer seasons and individuals were considered migratory if they made movements > 10 km from their capture location on leks to winter locations (Connelly et al., Reference Connelly, Schroeder, Sands and Braun2000). The distance to suitable winter habitat may be constrained by lek location and we stratified measurements for each lek where females were captured. We measured the distance individuals moved between consecutive flights to document movements within winter habitat and divided the measurement by the number of days between flights. We used a 100% minimum convex polygon to calculate the area encompassed by the outermost extent of radio-marked bird locations.
Juvenile sage-grouse may seasonally disperse further than adults in some landscapes (Dunn & Braun, Reference Dunn and Braun1985; Connelly et al., Reference Connelly, Browers and Gates1988; Beck et al., Reference Beck, Reese, Connelly and Lucia2006). We could not test for differences in movements between juveniles and adults because females captured at leks had already survived more than one winter. However, juveniles may still be imprinting on winter ranges and could make larger movements than adults if they are still seeking high quality habitat. We tested whether yearling and adults differed in mean distances moved from summer to wintering range, and within their winter range, to explore this hypothesis.
Results
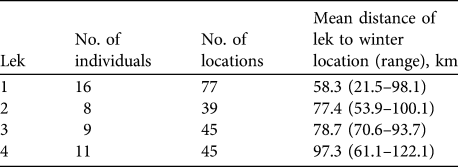
We obtained 209 locations from 39 individuals on 12 flights between 26 January 2008 and 3 March 2009. Each radio-marked individual moved > 21 km from summer to winter ranges and 122 km was the longest documented movement (Table 1). All but five of the 209 locations were south of the Milk River in big sagebrush habitat (Fig. 1). We recorded our last summer locations on 9 and 15 September in 2007 and 2008, respectively. All sage-grouse had migrated by our earliest winter flight on 17 November 2008 and were still on the winter range on 16 March 2009. We documented females attending leks north of the Milk River as early as 22 March 2008. Dispersal distances were similar between yearlings and adults and therefore we combined estimates by lek (Table 1). Distances migrated by lek differed because some leks were further north of the winter range. We also pooled individual movements between winter locations because yearling and adult movements were similar (P =0.31). We assumed movements between females from different leks were similar once individuals had reached winter habitat.
Table 1 Mean distance (with range) moved by radio-marked female greater sage-grouse Centrocercus urophasianus from four breeding leks (Fig. 1) to their winter range in north-eastern Montana and south-central Saskatchewan in 2007–2009.
Females on the winter range moved an average of 250 m per day, assuming straight-line uniform movements between flight intervals, with some movements estimated > 2.5 km per day. We relocated eight females more than two times each winter. Three of the eight females overlapped a portion of areas used in both winters and the remaining five females were located 1–25 km from the previous year’s location. Females mixed freely with individuals from other capture leks (Fig. 1). During flights we relocated several flocks that contained radio-marked females from multiple capture leks. The outermost extent of radio-marked bird locations recorded was 6,687 km2.
Discussion
The migratory movements we documented are probably annual obligate movements, not dependent on extreme winter weather events. We recorded all individuals moving > 20 km in consecutive seasons including a winter with the lowest snowfall recorded in 30 years (Opheim 12 SSE Weather Station, U.S. National Climatic Data Center). Migratory movements we observed are not a mechanism for dispersal because adult females returned to leks north of the Milk River in subsequent years. We cannot infer the same for males or juveniles because females captured at leks had already survived more than one winter. We may be missing an age- or sex-specific trait of dispersal by examining only females that survived more than one winter over 2 years. Genetic evidence suggests that populations north and south of the Milk River are distinct but a few individuals from south of the Milk River assigned to leks in Alberta, Canada (Bush et al., Reference Bush, Dyte, Moynahan, Aldridge, Sauls and Battazo2011).
Sage-grouse probably migrated because breeding areas lack sufficient sagebrush cover in winter. Females breeding in silver sagebrush habitats north of the Milk River used distinct areas on breeding and summer ranges (Fig. 1). Sage-grouse migrated to the winter range in big sagebrush habitat south of the Milk River by early November, where birds captured from different leks mixed freely with each other in wintering areas (Fig. 1). Sage-grouse in nearby Alberta, Canada, use silver sagebrush habitats in winter with high apparent survival (73–88%; J. Carpenter, pers. comm.). In the uplands of Alberta there are larger remaining tracts of silver sagebrush than in Saskatchewan.
We are concerned that expanding human development could degrade otherwise suitable winter habitat. Expanding agricultural tillage results in loss of sagebrush habitat and wintering sage-grouse avoid otherwise high quality winter habitat as well density from oil and gas development increases (Doherty et al., Reference Doherty, Naugle, Walker and Graham2008). Agricultural tillage continues to encroach upon sagebrush habitat along the Milk River and radio-marked females spent the winter near a developed portion of the Bowdoin gas field south of Hinsdale, Montana (Fig. 1). Winter habitat will be reduced if agricultural tillage continues along the Milk River or if oil and gas development expands into authorized leases south of U.S. Highway 2 (Fig. 1). Understanding how and when sage-grouse migrate is pivotal in understanding the mechanisms of large movements and the role of transitional habitat in facilitating long and presumably costly movements. Maintaining connectivity between seasonal ranges requires knowing if and how sage-grouse use transitional habitats. Habitat use along migratory pathways remains unknown because VHF technology cannot keep pace with the timing and distance of migratory movements.
New GPS technology provides the ability to identify potential habitat pathways between seasonal ranges. Large deciduous trees line the banks of the Milk River and there is a c. 10-km wide strip of agricultural tillage running the length of the river (Fig. 1), both inhospitable habitats to the sagebrush-dependent sage-grouse (Doherty et al., Reference Doherty, Naugle, Walker and Graham2008). If there are corridors that sage-grouse rely upon to connect summer and winter habitats, these may be at risk from conversion to agriculture or increased development of oil and gas fields (Fig. 1). Identifying potential bottlenecks that restrict movement will be paramount to conserving this unique migratory event. Migratory movements add urgency to maintaining populations that transcend international boundaries because sage-grouse are an endangered species in Canada under the federal Species at Risk Act.
Extraordinary movements point to an important wintering area for sage-grouse that is a high priority for transboundary conservation. The winter range we observed is probably used by a large population of sage-grouse south of the Milk River, an area with some of the highest sage-grouse densities in their eastern range (Doherty et al., 2010). Our winter locations overlapped leks in big sagebrush, and several times we observed radio-marked individuals in flocks of > 100 birds, abundances probably higher than the entire Saskatchewan population of sage-grouse. Conservation of sage-grouse habitat in Montana will in part affect the viability of endangered sage-grouse in Canada.
The number of species that make disproportionately large movements has initiated a rethink regarding the land area necessary to conserve biodiversity in the northern Great Plains. The migratory sage-grouse population we studied encompasses 6,687 km2, an area 30 times larger than the East Block of Grasslands National Park (218 km2; Fig. 1). Similarly, reintroduced swift fox Vulpes velox dispersal distances (190 km) in Montana, Alberta and Saskatchewan (Ausband & Moehrenschlager, Reference Ausband and Moehrenschlager2009) exceed those of other populations, prairie rattlesnakes Crotalus viridis viridis in south central Alberta migrate further (> 52 km) than any other terrestrial snake (Jorgensen et al., Reference Jorgensen, Gates, Whiteside, Hayes, Cardwell, Beamen and Bush2008), and pronghorn regularly migrate > 100 km between Saskatchewan and Montana (A. Jakes, unpubl. data). Science can help decision makers identify the size and location of landscapes to prioritize for conservation. Nevertheless, the fate of remaining large and intact prairie landscapes ultimately depends on our ability to foster partnerships that result in implementation of long-term conservation.
Acknowledgements
Matt Tribby provided invaluable help in the field. Funding and support was provided by the U.S. Bureau of Land Management in Montana, Grasslands National Park of Canada, and Steve Forrest of WWF. Additional project support came from the University of Montana, and Montana Fish, Wildlife and Parks. We appreciate comments from J.W. Connelly and C.E. Braun that helped strengthen this article. The views in this article are those of the authors and do not necessarily reflect those of their employers.
Biographical sketches
Jason Tack has worked on sage-grouse habitat and conservation issues for the past 4 years in Montana and Saskatchewan, and David Naugle’s applied research focuses on creating conservation planning tools for focal species in the mid continent and western grasslands of the USA. Together, they are creating conservation planning tools for sage-grouse populations range-wide. John Carlson is a wildlife biologist and Pat Fargey a species-at-risk biologist, and for the past 10 years they have been helping guide research to manage effectively transboundary conservation issues in the northern Great Plains.