INTRODUCTION
Staphylococcus aureus is an opportunistic pathogen that is a frequent cause of infections in both the hospital and the community. The anterior nares are the most common colonization site for S. aureus and colonization with S. aureus is a risk factor for the development of subsequent infection [Reference Wertheim1]. Most often these infections are due to the patient's endogenous or colonizing isolate [Reference Wertheim1, Reference Wertheim2]. The relationship between colonization and subsequent S. aureus infection has been studied in multiple patient populations, including extended-care residents [Reference Muder3–Reference Bradley7]. The risk of developing an infection among methicillin-resistant S. aureus (MRSA) colonized patients is 4–20 times greater than that of methicillin-sensitive S. aureus (MSSA)-colonized or non-colonized patients [Reference Muder3–Reference Bradley7].
MRSA was predominantly a hospital-acquired pathogen, seen primarily in people in contact with the healthcare setting. In the last two decades, MRSA has emerged as the most common cause of skin and soft tissue infections in the community and most of these infections are due to a single clone, USA300 MRSA [Reference Moran8–Reference Johnson10]. Furthermore, USA300 MRSA is now also common in the healthcare setting causing hospital-associated infections [Reference Klevens11].
A number of investigators have hypothesized that USA300 MRSA skin and soft tissue infections have a different pathogenesis than other S. aureus infections and may occur without preceding colonization [Reference Miller and Diep12]. Nasal colonization with USA300 MRSA occurs at a lower frequency than other S. aureus isolates including non-USA300 MRSA in patients with community-acquired skin and soft tissue infection [Reference Frazee13, Reference Kazakova14]; however, we have shown that nasal colonization with USA300 MRSA is similar to nasal colonization with non-USA300 MRSA in a long-term care population [Reference Shurland15]. To further examine the pathogenesis of USA300 MRSA infection in long-term care residents, we assessed the risk of subsequent MRSA infection in extended-care residents colonized with USA300 MRSA compared to residents not colonized with MRSA or residents colonized with non-USA300 MRSA.
METHODS
Study setting
The study was conducted in five extended-care units in two geographically separate facilities that are part of the Veterans Affairs Maryland Health Care System (VAMHCS). The VAMHCS provides comprehensive healthcare to over 45 000 veterans in the Maryland area. Patients admitted to these facilities receive short-stay or long-stay services for rehabilitation, skilled nursing care or maintenance care. This is similar to the type of care received at non-VA long-term care facilities in the USA. Newly admitted or re-admitted residents to the extended-care units are screened for MRSA within 1 week of admission. Surveillance for MRSA colonization is routinely performed on these extended-care units every 3 months. Residents with MRSA colonization often receive a one-time decolonization regimen consisting of 5-day treatment course with mupirocin nasal ointment twice a day and chlorhexidine baths on days 1, 3 and 5 of mupirocin. This protocol was used at the discretion of the attending physician. The University of Maryland Baltimore Institutional Review Board and the VAMHCS Research and Development Committee approved this study.
Study population and design
We performed a retrospective cohort study of extended-care residents that had at least one surveillance culture of the anterior nares for MRSA between 1 April 2003 and 30 November 2007. Eligible residents met the following criteria: (1) were aged ⩾18 years, (2) admitted to the VAMHCS extended-care units for more than 1 week, (3) had at least one surveillance culture performed during the study period, and (4) had to have their MRSA isolate available for molecular testing if they were MRSA colonized. We excluded residents admitted to the extended-care unit for treatment of a MRSA infection.
Culture techniques
All cultures were processed using standard microbiological methods in the VAMHCS clinical microbiology laboratory. S. aureus isolates were characterized by catalase and coagulase production (Staphaurex, Remel, USA). MRSA isolates were determined by growth on oxacillin (6 μg/ml) agar screen plates incubated at 37°C and read within 48 h. The first MRSA isolate per resident was frozen at −70°C in tryptic soy broth with glycerol.
Molecular methods
MRSA isolates were re-grown on a 5% blood agar plate for DNA extraction. Chromosomal DNA was extracted from cells after growth in an overnight culture of tryptic soy broth at 37°C. The cell suspension was lysed using a 1 in 5 μl solution of lysostaphin to cell suspension for 2–3 h incubation at 37°C. DNA isolation was performed using the Prepman Ultra kit (Applied Biosystems Inc., USA) according to the manufacturer's instructions. All MRSA isolates were characterized using previously described primers to amplify Panton–Valentine leukocidin (PVL), arginine catabolic metabolic element (ACME) and the polymorphic region of the protein A (spa) genes [Reference Lina16–Reference Harmsen18].
Study variable
If MRSA was detected on a surveillance culture, the resident was classified as colonized with USA300 MRSA or non-USA300 MRSA. Residents were classified as colonized with USA300 MRSA if their MRSA isolate had the spa-type motif MBQBLO, and was PVL and ACME positive. All other types were categorized as non-USA300 MRSA. The validity of this algorithm has been reported previously [Reference Kreisel19]. Colonization with MRSA had to precede MRSA infection. If MRSA was not detected in any anterior nares culture during the period of extended-care unit stay, the resident was classified as not colonized with MRSA.
Outcome variable
All study residents were followed from the time of study start or extended-care admission for 1 year or until the study ended in November, 2007. Thus the maximum time a resident was followed for MRSA infection was 1 year. This follow-up occurred even if the study subject was discharged from the long-term care facility through review of their microbiology culture records. The electronic medical records of all study residents with a clinical culture positive for MRSA were evaluated using previously defined standard criteria to determine whether the infection was due to MRSA in extended-care residents [Reference McGeer20]. For example, the electronic medical record of a resident with a wound culture positive for MRSA was reviewed to determine whether the criteria for a skin and soft tissue infection were met. Only the first episode of MRSA infection in a resident was considered in the statistical analysis. We defined invasive infection by the isolation of MRSA from a normally sterile site which included blood, cerebrospinal fluid, pleural fluid, pericardial fluid, joint/synovial fluid.
We collected demographic information such as age, sex and race from the electronic medical record. Length of extended-care stay (i.e. inpatient extended-care stay) and level of care were also abstracted from medical records. Underlying conditions such as diabetes, HIV infection and renal replacement therapy were taken using the International Classification of Diseases, 9th edition (ICD-9) codes from discharges that occurred during the follow-up period. The Charlson comorbidity index was calculated for each patient so that it represented the patient's condition at the time of admission on the basis of comorbidities listed in ICD-9 codes as adapted by Deyo et al. [Reference Deyo, Cherkin and Ciol21]. The index encompasses 19 medical conditions and a weighted score from 1 to 6 was assigned with total scores ranging from 0 to 37. Skin breakdown from pressure ulcers and chronic ulcers during the 1-year follow-up period were determined from discharge coding as described previously [Reference Roghmann22]. Surgery performed and the presence of central venous catheters during the 1-year follow-up period were identified from clinical procedure codes.
Statistical analysis
Data analysis was based on testing the hypothesis that residents colonized with USA300 MRSA have a greater risk of infection compared to residents not colonized with MRSA or residents colonized with non-USA300 MRSA. Continuous variables were compared by using the Student's t test or Wilcoxon test as appropriate. Categorical variables were compared using the χ2 test or Fisher's exact test, as appropriate. Relative risks with their 95% confidence intervals were calculated by comparison to reference categories. Cumulative Kaplan–Meier plots were constructed with entrance into the study as the starting point and the first episode of MRSA infection as the end point. Effect-modifying variables were assessed by creating interaction terms between the main study variable and the potential effect modifiers in a Cox regression model. Confounding variables were assessed by a change of 10% or more between the crude hazard ratio and adjusted hazard ratio controlling for the potential confounder. Variables found to be significantly associated with the infection or important based on literature review were also entered into a multivariable Cox proportional hazards model. We used graphical methods to check the proportional hazards assumption. A multivariable Cox proportional hazards model assessed the effect of USA300 MRSA colonization on the hazard of infection adjusting for identified potential confounding and effect-modifying variables and calculating the adjusted hazard ratios (relative risks) and 95% confidence intervals. All statistical tests were two-sided, and P values <0·05 were considered significant. Statistical analyses were performed using SAS statistical software version 9.1 (USA).
RESULTS
Description of the study population
During the 4-year period from April 2003 to November 2007, a total of 2614 residents were admitted to the extended-care units in the VAMHCS, of which 1691 (65%) met our inclusion criteria. Of the 923 residents that were excluded, 267 were excluded because their length of stay was <1 week, 556 had no surveillance culture performed, 50 had an active MRSA infection at the time of admission and 50 had MRSA isolates that were not available for molecular testing (Fig. 1). Residents that did not have a surveillance culture performed were significantly more likely to be older (73±13 years vs. 68±13years, P<0·01), have a shorter length of extended-care stay (117±254 days vs. 187±427 days, P<0·01), and were in residential care.
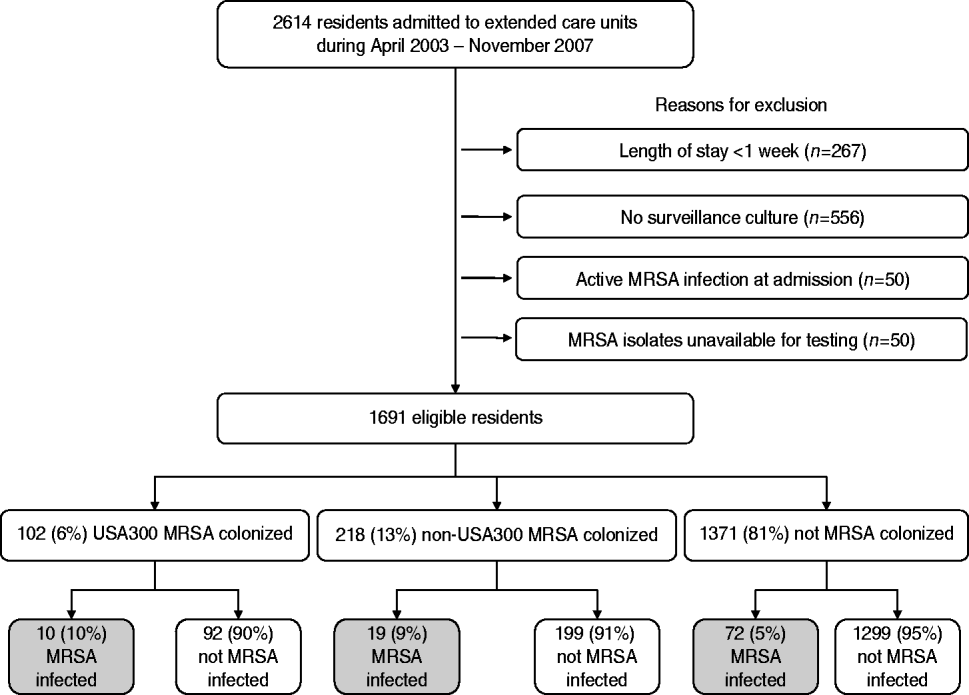
Fig. 1. Study flow diagram.
Our eligible study population had a mean age of 69 years and most residents were male (96%). Fifty-three per cent of residents were white, 46% were African American and 1% were of other or mixed race. Most residents were in residential care (46%). Diabetes (34%) was the most common underlying condition. Very few residents had renal replacement therapy (2%) or HIV infection (2%).
MRSA colonization
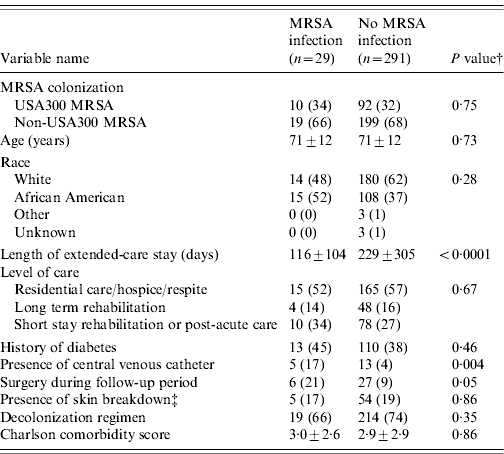
Of the 1691 residents, 320 (19%) were colonized with MRSA and 1371 (81%) residents were not colonized. USA300 MRSA accounted for 102 of the 320 residents colonized with MRSA (see Fig. 1). Table 1 compares the demographic and clinical characteristics of these residents. When compared to residents not colonized with MRSA, residents colonized with USA300 MRSA were more likely to be in long-term rehabilitation, have diabetes, skin breakdown or a central venous catheter during the 1-year follow-up period. When compared to residents colonized with non-USA300 MRSA, colonization with USA300 MRSA was significantly associated with younger age, being African American and having a central venous catheter during the 1-year follow-up period. USA300 MRSA-colonized residents were similar to non-USA300 MRSA-colonized residents with respect to length of stay, underlying comorbidities as measured by the adapted Charlson comorbidity index and presence of skin breakdown.
Table 1. Comparison of colonization patterns in extended-care residents in the VAMHCS from 2003 to 2007 (n=1691)Footnote *
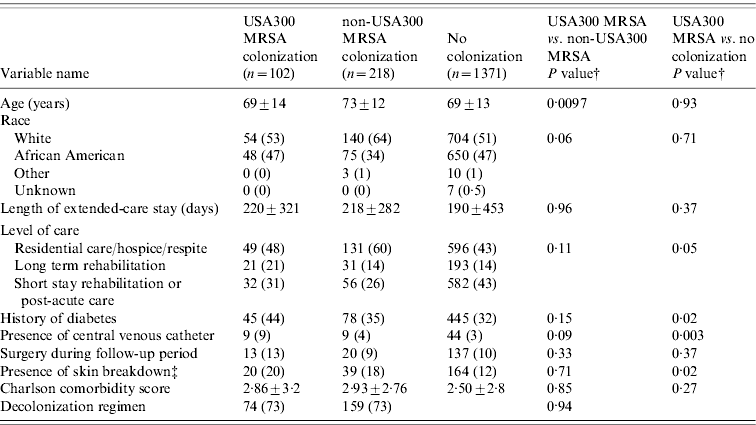
VAMHCS, Veterans Affairs Maryland Health Care System.
* Categorical data are n (column %), continuous data are mean±s.d.
† P values from χ2 test for categorical variables or Student's t test for continuous variables.
‡ Presence of skin breakdown=pressure, chronic and decubiti ulcers.
Types of MRSA infections
There were 101 MRSA infections detected during the 1-year follow-up for each eligible resident (see Fig. 1). Ten (10%) of the 101 residents who were colonized with USA300 MRSA developed a MRSA infection compared to 72 (5%) of 1314 residents who were not colonized with MRSA. Nineteen (9%) of the 218 residents who were colonized with non-USA300 MRSA developed a MRSA infection. Skin and soft tissue infections were the most common infection type in all groups. Twenty percent of the infections were invasive; none of the USA300 MRSA-colonized residents had an invasive infection.
Risk factors for MRSA infection
Table 2 compares demographic and clinical characteristics in residents that developed a MRSA infection among USA300 MRSA-colonized residents and non-colonized residents. Residents colonized with USA300 MRSA had a twofold increased risk of developing a MRSA infection compared to residents not colonized with MRSA [hazard ratio (HR) 1·87, 95% confidence interval (CI) 1·0–3·5, P=0·05] (Fig. 2). Residents who had a MRSA infection were more likely to have a long-term rehabilitation stay, history of diabetes, renal replacement therapy, presence of central venous catheter, surgery performed, skin breakdown, and a higher Charlson comorbidity index score (Table 2).
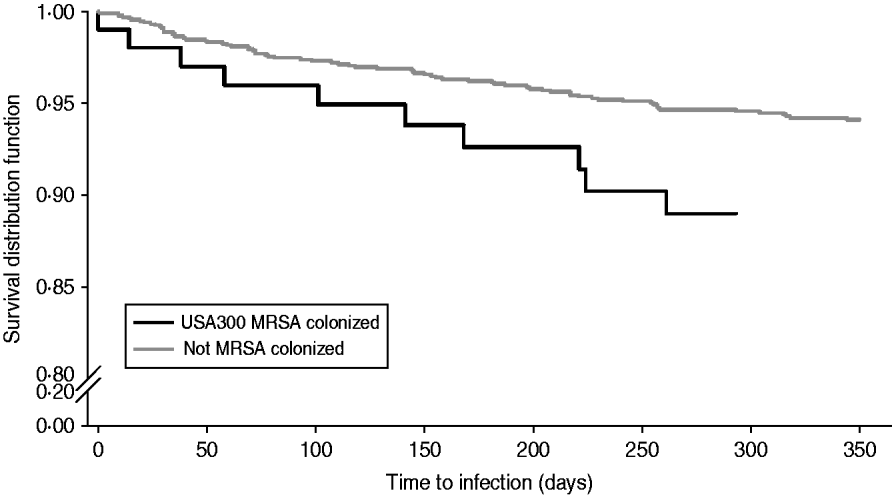
Fig. 2. Kaplan–Meier plots of the risk of MRSA infection in USA300 MRSA-colonized compared to non-colonized extended-care residents. Log rank P value <0·05.
Table 2. Risk factors associated with the development of a MRSA infection in USA300 MRSA-colonized and not colonized MRSA extended-care residents in the VAMHCS from 2003 to 2007 (n=1473)Footnote *
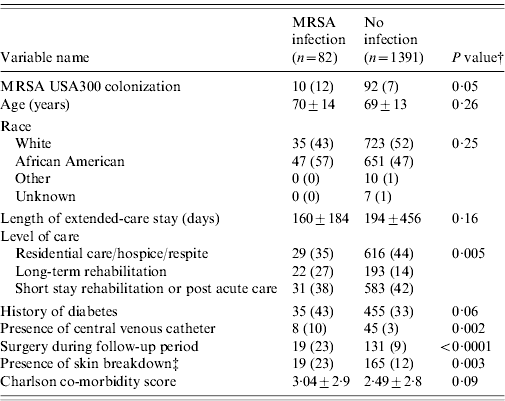
VAMHCS, Veterans Affairs Maryland Health Care System.
* Categorical data are n (column %), continuous data are mean±s.d.
† P values from χ2 test for categorical variables or Student's t test for continuous variables.
‡ Presence of skin breakdown includes pressure, chronic and decubiti ulcers.
Table 3 compares demographic and clinical characteristics in residents that developed a MRSA infection among USA300 MRSA-colonized residents and non-USA300 MRSA-colonized residents. In this population, there was no difference in the risk of developing a MRSA infection when residents colonized with USA300 MRSA were compared to residents colonized with non-USA300 MRSA (HR 1·12, 95% CI 0·5–2·3, P=0·80) (Fig. 3). Residents who had a MRSA infection were more likely to have a shorter length of stay, a central venous catheter and surgery performed within the 1-year follow-up (Table 3). We did not detect a difference in the risk of MRSA infection in those who received the decolonization regimen compared to those who did not (relative risk 0·70, 95% CI 0·34–1·46).
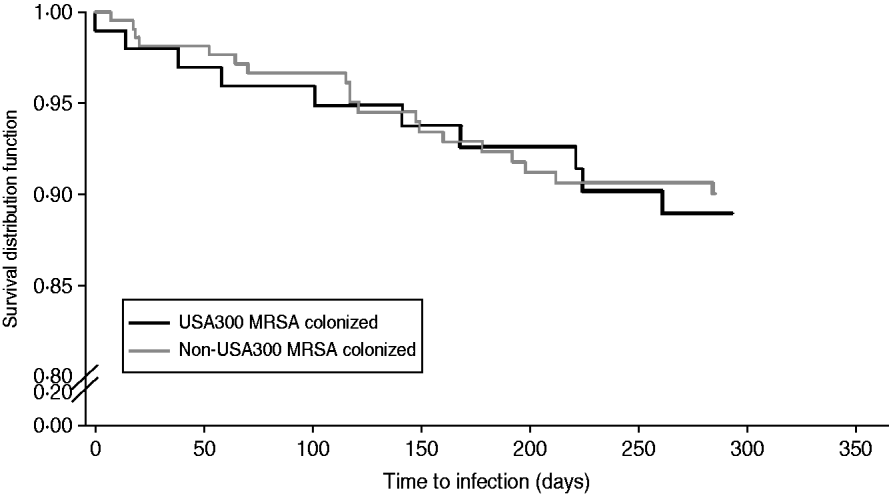
Fig. 3. Kaplan–Meier plots of the risk MRSA infection in USA300 MRSA-colonized compared to non-USA300 MRSA colonized extended-care residents. Log rank P value >0·05.
Table 3. Risk factors associated with the development of a MRSA infection among only MRSA colonized extended-care residents in the VAMHCS from 2003 to 2007 (n=320)Footnote *
VAMHCS, Veterans Affairs Maryland Health Care System.
* Categorical data are n (column %), continuous data are mean±s.d.
† P values from χ2 test for categorical variables or Student's t test for continuous variables.
‡ Presence of skin breakdown includes pressure, chronic and decubiti ulcers.
Stratified and multivariable analysis
When residents colonized with USA300 MRSA were compared to residents not colonized with MRSA, stratified analysis showed that surgery performed during the 1-year follow-up period was the only variable that modified the effect of USA300 MRSA colonization and the risk of infection. Of residents that did not have surgery, the risk of MRSA infection in residents colonized with USA300 MRSA was 2·3 times greater than the risk of MRSA infection in residents not colonized with MRSA (HR 2·3, 95% CI 1·2–4·5, P=0·03). Of residents that did have surgery, the risk of MRSA infection was not significantly different between residents colonized with USA300 MRSA and residents not colonized with MRSA (HR 0·6, 95% CI 0·1–4·0, P=1·00). There were no variables that potentially confounded the association among USA300 MRSA colonization and the risk of MRSA infection. Multivariable analysis showed that in residents that did not have surgery, the risk of infection among USA300 MRSA-colonized residents was significantly higher compared to residents not colonized with MRSA (HR 2·3, 95% CI 1·1–4·7, P=0·02) after adjusting for factors associated with MRSA infection including underlying comorbidities as measured by the Charlson score (HR 1·09, 95% CI 1·0–1·2, P=0·01), presence of skin breakdown (HR 1·93, 95% CI 1·0–3·6, P=0·04) and patient setting (i.e. rehabilitation care vs. residential care) (HR 1·58, 95% CI 0·9–2·6, P=0·08).
When residents colonized with USA300 MRSA were compared to residents colonized with non-USA300 MRSA, stratified analysis showed no variables including receipt of the decolonization regimen that modified the effect of USA300 MRSA colonization and the risk of infection (data not shown). There were no variables including receipt of the decolonization regimen that potentially confounded the association among residents colonized with USA300 MRSA and the risk of MRSA infection. Thus further multivariable analysis was not performed.
DISCUSSION
The principal tenet in the pathogenesis of S. aureus infections, including MRSA infections, is that colonization precedes infection and the infection is due to the individual's endogenous strain. A number of investigators have hypothesized that USA300 MRSA skin and soft tissue infection may arise without preceding colonization rather than a stepwise progression of exposure to MRSA, followed by colonization and then infection [Reference Miller and Diep12].
We assessed the role of USA300 MRSA nasal colonization and the risk of subsequent MRSA infection in extended-care facilities and compared the risk of MRSA infection in residents not colonized with MRSA and residents colonized with non-USA300 MRSA. In this study, we found that the risk of MRSA infection was twofold higher in extended-care residents who were colonized with USA300 MRSA compared to residents not colonized with MRSA. The magnitude of the relative risk of MRSA infection in patients with MRSA colonization is consistent with other studies. Relative risks ranging from 3 to 26 have been reported [Reference Wertheim2–Reference Mest4, Reference Bradley7, Reference Pujol23, Reference Davis24]. Muder et al. showed that 25% of rehabilitation and long-term-care residents colonized with MRSA developed MRSA infections compared to 4% of those not colonized with MRSA [Reference Muder3]. Bradley et al. also showed that MRSA colonization at admission increased the risk of subsequent infection more than sixfold compared to residents not colonized with MRSA [Reference Bradley7]. Our findings suggest that USA300 colonization precedes infection and increases the risk of infection as seen with non-USA300 MRSA.
The USA300 MRSA genotype carries unique genetic characteristics that distinguish it from non-USA300 MRSA including PVL and ACME. It has been hypothesized that USA300 MRSA may be more virulent compared to non-USA300 MRSA based on these genetic differences. Although our sample size is limited, we found no significant difference in the risk of infection in residents colonized with USA300 MRSA compared to non-USA300 MRSA residents colonized in the extended-care setting. Since the results of our study suggest no difference in the risk of infection, host characteristics (e.g. immune response to USA300 MRSA) in combination with environment factors (e.g. physical contact) may be responsible for the epidemic of USA300 MRSA infections in the community and should be further investigated.
Host-related factors such as surgical incisions, central venous catheters, pressure ulcers, and underlying co-morbid conditions are known to increase the risk of MRSA infections. We confirmed these risk factors in our extended-care facility residents colonized with USA300 MRSA. We did not find a protective effect of a decolonization regimen on the risk of MRSA infection although this study was not designed to test the effectiveness of this regimen.
Our study has a number of limitations. It is limited by its retrospective nature and the fact that laboratory procedures for storage of MRSA isolates influenced which isolates were available for typing. We only stored the first MRSA isolate per patient and those from research studies thus limiting the number of MRSA isolates available for molecular typing from each resident. When more than one isolate was available, we chose the isolate closest to the study period. We measured colonization status based on surveillance cultures of the anterior nares and a number of papers have reported that nasal colonization is less common with USA300 MRSA [Reference Yang25, Reference Peters26]. Despite this, we do not believe that we underestimated colonization with USA300 MRSA compared to non-USA300 MRSA because our prior work in this population found that the sensitivity of the anterior nares for detecting USA300 MRSA was similar to its sensitivity for non-USA300 MRSA [Reference Shurland15]. In addition, we did not have MRSA isolates from the infections and thus colonizing isolates could not be explicitly linked to the isolates causing infection. We used spa-type motif and the presence of the PVL and ACME genes to characterize isolates as USA300. Although it is uncommon, we may have misclassified some USA300 MRSA isolates as non-USA300 if these genes were lost as has been reported recently [Reference Gorwitz27–Reference Ellis29]. The overall number of MRSA infections was small which limits the precision of the calculated hazard ratios and confidence intervals. Finally, our study population consisted of veterans in extended-care facilities. This limits our generalizability to other populations particularly community-dwelling adults with USA300 MRSA infections.
Our study has a number of strengths. This is the first study that has assessed the role of USA300 MRSA nasal colonization and subsequent MRSA infection. Other studies have assessed colonization at the time a MRSA infection was diagnosed [Reference Frazee13, Reference Schechter-Perkins30]. We used molecular techniques to determine the MRSA genotype which gives a more accurate assessment of the residents' colonization status than imputing the genotype from other characteristics.
USA300 MRSA is an emerging pathogen and its epidemiology and pathogenesis is incompletely understood. Colonization with MRSA has already been described as a risk factor for subsequent infections in many populations, and frequently infections are due to endogenous or colonizing organisms. We found the risk of infection in USA300 MRSA-colonized residents is significantly higher compared to residents not colonized with MRSA. No difference was found in the risk of infection in residents colonized with USA300 MRSA compared to residents colonized with non-USA300 MRSA. The growing proportion of USA300 MRSA infections warrants more studies in different study populations (e.g. health-community-dwelling children or adults) to determine the importance of USA300 MRSA colonization as well as host and environmental characteristics on the risk of developing a USA300 MRSA infection. These results will be important for developing new prevention strategies for USA300 MRSA infections.
ACKNOWLEDGEMENTS
Dr M.-C. Roghmann supported this work by her VA Research and Development Merit Award and the University of Maryland School of Medicine General Clinical Research Center (M01-RR-16500). Dr J.P. Furuno was supported by the Multidisciplinary Clinical Research Career Development Program (1K12RR023250) while this study was performed. Dr R. Miller is supported by a mentored patient-oriented research career development award from the NIH (1K23AG027746).
DECLARATION OF INTEREST
None.










