The epidemic of obesity continues to grow throughout the world(1). Estimates suggest that the direct cost of obesity accounts for approximately 7 % of total health care(2). Lowering dietary energy density has been suggested as an effective strategy to prevent and treat obesity(2–Reference Rolls4). More ambitiously, James(Reference James5) has proposed a full transformation of the food system to one of nutritious, low-energy-dense foods.
Nuts are considered as an energy-dense food, having an estimated 23–30 kJ/g compared with an energy density of 7·5 kJ/g for a typical diet overall(Reference Rolls4). Thus, inclusion of nuts in the diet would be counter to the recommendations to reduce dietary energy density. However, nuts convey various important health benefits. Studies have shown that nuts reduce risk factors associated with CVD in clinical studies(Reference Sabate, Haddad and Tanzman6–Reference Gebauer, West and Kay8), lower risk factors or incidence of cardiovascular events in epidemiological studies(Reference Fraser, Sabate and Beeson9, Reference Hu, Stampfer and Manson10), reduce glycaemic index of a composite meal(Reference Josse, Kendall and Augustin11), lower risk of diabetes(Reference Jiang, Manson and Stampfer12), improve glucose metabolism(Reference Jenkins, Kendall and Marchie13, Reference Sari, Baltaci and Bagci14) and possibly lower cancer risk(Reference Davis and Iwahashi15, Reference Gonzalez and Salas-Salvado16). Thus, excluding nuts from the diet to lower energy density withholds key health benefits that nuts provide.
Epidemiological studies have suggested that individuals consuming nuts regularly do not suffer from higher body weights or BMI values, but rather there is an inverse association between frequent nut consumption and BMI(Reference Hu, Stampfer and Manson10, Reference Casas-Agustench, Bulló and Ros17). In addition to epidemiological associations, clinical studies indicate that incorporation of nuts into an otherwise ad libitum diet promotes little to no weight gain(Reference Morgan and Clayshulte18–Reference Hollis and Mattes23). Clinical studies also indicate that lipid from nuts is poorly absorbed, based on postprandial lipaemia and appearance of fat in faecal samples(Reference Levine and Silvis24–Reference Casas-Agustench, Lopez-Uriarte and Bullo27). It follows then that nuts impart less dietary energy than the value calculated from the Atwater general factors, though this has never been addressed in a feeding study. This study was conducted to determine the energy value of nuts incorporated into an otherwise balanced diet.
Experimental methods
Subjects, study design and diet
A convenience sample of men and women was recruited from the area surrounding the Beltsville Human Nutrition Research Center (Beltsville, MD, USA) to participate in a feeding study. Volunteers were healthy non-smokers with no malabsorption or gastrointestinal disorders and no history of bariatric surgery. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the MedStar Health Research Institute Committee on Human Research (Hyattsville, MD, USA). Written informed consent was obtained from all the subjects.
A total of eighteen subjects (nine men, nine women) were selected to participate in a dose–response, randomised crossover controlled feeding trial (all food items weighed to the nearest gram amount). Three doses of pistachios were fed as part of a controlled diet with each dose fed for 18 d. The doses studied were the following: (1) 0 g/d (control), (2) 42 g/d (equal to one serving per d as defined by the US Food and Drug Administration) and (3) 84 g/d (equal to two servings per d). The pistachios were incorporated into a controlled diet with a proportionate reduction of all foods in the controlled diet to maintain isocaloric intake across treatments. Subjects were stratified by sex and initial BMI and randomly assigned to a treatment sequence. Each sequence included two dose levels (0, 1·5 or 3 oz/d) and a repetition of one of the doses, and the dose sequence was randomly assigned by a statistician.
Study subjects were fed at weight maintenance. Body weight was measured, before breakfast, Monday to Friday, to identify patterns of weight loss or weight gain over 7- to 10-d periods. If patterns of weight change were observed, portion size was adjusted for all foods (in 837 kJ increments) to maintain weight. No diet adjustments were made during the balance collection periods. Volunteers were fed traditional American foods incorporated into a constant 7-d menu cycle and were instructed to consume all the food and only the food provided by the Beltsville Human Nutrition Research Center. Breakfast and dinner were consumed at the Nutrition Center, and lunch and weekend foods were packed for carry-out. Breakfast and dinner on weekdays, including pistachio treatments, were consumed under the observation of a dietitian, research associate or investigator to verify compliance. Subjects completed questionnaires daily to report any breeches in compliance, and study staff regularly engaged subjects in conversations about compliance. The fat:fibre ratio of the background diet was matched to that of the nuts so that it remained constant over all treatments, since fibre has been shown to influence fat absorption(Reference Baer, Rumpler and Miles28).
Biological sample collection and chemical analyses
Faecal collections were initiated on the 9th day after the treatment began and subjects started consuming diet. To mark the beginning of the faecal collection period, we administered, at dinner, a capsule containing approximately 15 mg brilliant blue. After the subjects consumed the capsule, they were instructed to collect all of their faeces. After 7 d, a second capsule of brilliant blue was administered at dinner, and subjects were instructed to continue collecting their faeces until they were told to stop by a study investigator (once the appearance of the marker in faeces was observed by study staff). The brilliant blue marker was used to determine the beginning and end points of the faecal balance period. Faecal samples were stored in a cooler with dry ice until delivery to the Center, usually within 12 h of collection. Once received at the Center, faecal samples were weighed (wet weight) and placed in a freezer until they were freeze-dried. Immediately after they were removed from the freeze-drier, the samples were weighed (dry weight), and then pulverised using a food processor to produce a homogeneous powder. Diets were collected and prepared for chemical analysis by homogenising the food in a blender with ice and water before being freeze-dried.
Urine was collected in pre-weighed 4-liter containers with 15 g boric acid. The containers were kept on ice and delivered to the Center each morning. Each day, subjects received new containers. At the Center, samples were weighed and subsamples were collected and stored at − 80 °C until analyses were performed. The weight of the urine voided was calculated as the difference between the full container weight and the pre-tare empty container weight.
Diets, faeces and urine were analysed for combustible energy by adiabatic bomb calorimetry (Parr Instrument Company, Moline, IL, USA) and for N by combustion (Leco, St. Joseph, MI, USA). Diets and faeces also were analysed for fat (petroleum extraction; Foss, Eden Prairie, MN, USA), total dietary fibre (Foss) and ash (muffle furnace). All analyses were carried out in duplicate, and the mean of these values was used for statistical analyses.
Blood samples were collected at the end of each treatment period. Blood was collected from an antecubital vein using an appropriate-sized needle into an evacuated sterile tube containing EDTA. Following collection, the tubes were centrifuged and the plasma was collected, aliquoted and frozen at − 80 °C until analyses. Plasma total cholesterol, direct LDL-cholesterol, HDL-cholesterol and TAG levels were measured using enzymatic photometric methods (Dimension Xpand; Dade-Behring, Deerfield, IL, USA).
Calculations
Total carbohydrate (DM basis) was calculated as follows:
We derived the following equation for the metabolisable energy (ME) value of pistachios from the relationship that the ME of the whole diet is equal to the sum of the ME values of the parts:
where GEI is the gross energy intake (kJ/d) and MEI the ME intake (kJ/d). MEI is calculated as the difference between GEI and faecal (FE) and urinary energy (UE) output (MEI = GEI − (FE+UE)).
Nutrient digestibility was calculated as follows:
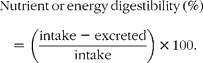
The currently accepted energy value of pistachios, as found on nutrient labels and in the USDA National Nutrient Database for Standard Reference (US Department of Agriculture, Agricultural Research Service, 2009. USDA National Nutrient Database for Standard Reference, Release 22. Nutrient Data Laboratory Home Page, http://www.ars.usda.gov/nutrientdata), is based on the Atwater general factors for the energy density of fat, protein and carbohydrate. The Atwater factors for fat, protein and carbohydrate are 37·7, 16·7 and 16·7 kJ/g respectively.
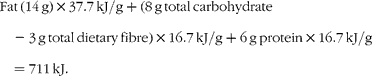
.
A 30 g serving of pistachios provides 14 g fat, 8 g total carbohydrate (including 3 g total dietary fibre) and 6 g protein. Note that current US Food and Drug Administration regulations allow insoluble fibre to be assigned an energy density of zero, thus the contribution of fibre has been subtracted from the total carbohydrate in the equation given later. Based on this information, the calculated energy density of pistachios is 711 kJ/30 g, yielding an energy density of 23·7 kJ/g.
Statistical analysis
To determine the effect of the treatment on wet weight, dry weight, number of bowel movements and chemical composition of the diet and excreta, we performed a mixed-model repeated-measures ANOVA using the subject as the random term (SAS version 9; SAS Institute, Inc., Cary, NC, USA). The statistical model included terms for treatment and period and interaction terms for subject × treatment. Results are reported as least-square means and sem.
Results
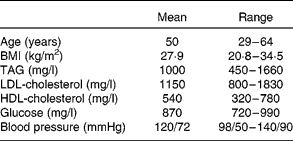
Of the eighteen subjects who began the study, sixteen completed the entire study protocol. Two subjects failed to complete the study due to schedule conflicts. Physical characteristics of the sixteen subjects are reported in Table 1. There were no self-reported side effects of the treatment. No major compliance issues were detected.
Table 1 Physical characteristics of eight male and eight female subjects who completed the intervention
(Mean values and ranges)
The food intake (dry weight) and N intake did not differ among treatments (Table 2). With the addition of pistachios and concomitant reduction of the base diet, there was an increase in the daily intake of fat and total dietary fibre with a concomitant reduction in total carbohydrate. These changes were significant between the control and 1·5 oz/d treatments as well as between the 1·5 oz/d and the 3 oz/d treatments. There was a small increase in GEI between the control and 3 oz/d treatments (452 kJ/d), whereas the energy intake of the 1·5 oz/d treatment was intermediate to the two other treatments and not different from either of the other treatments.
Table 2 Daily nutrient and energy intake
(Mean values with their standard errors)
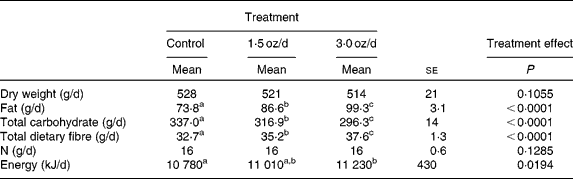
a,b,c Mean values within a row with unlike superscript letters were significantly different.
Total faecal wet weight increased from the control to the 1·5 oz/d treatment and was not different for the 1·5 oz/d compared with the 3 oz/d treatment (Table 3). Faecal dry weight increased from the control to the 1·5 oz/d treatment and further increased from the 1·5 oz/d to the 3 oz/d treatment. Faecal fat increased from the control to the 1·5 oz/d treatment and did not further increase from the 1·5 oz/d to the 3 oz/d treatment. Faecal energy output increased from the control to the 1·5 oz/d treatment and further increased from the 1·5 oz/d to the 3 oz/d treatment. Faecal total carbohydrate and total dietary fibre were similar among treatments. There was no effect of treatment on the number of bowel movements during the 7-d collection period.
Table 3 Bowel movements and faecal composition
(Mean values with their standard errors)
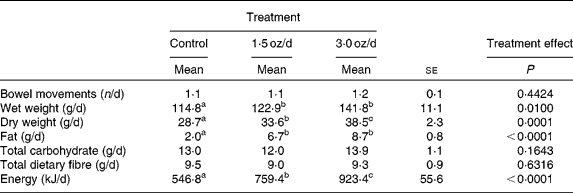
a,b,c Mean values within a row with unlike superscript letters were significantly different.
The digestibility of fat and energy decreased with the addition of pistachios to the diet (Table 4). The dose size of pistachios did not affect digestibility of fat or energy, as these values were similar between the 1·5 and 3 oz/d treatments. Total dietary fibre digestibility increased with the addition of pistachios, and the increase was significant for the 3 oz/d treatment compared to the control. The 1·5 oz/d treatment was intermediate to the control and 3 oz/d treatment.
Table 4 Nutrient and energy digestibility
(Mean values with their standard errors)
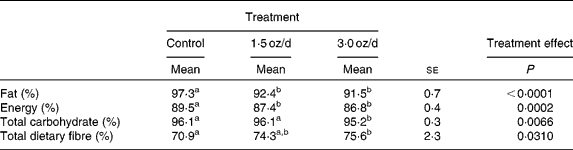
a,b Mean values within a row with unlike superscript letters were significantly different.
Based on the measurements collected in this study, the ME value of pistachios is calculated to be 22·6 kJ/g. This is lower than the value of 23·7 kJ/g as calculated by the Atwater general factors. Among individuals, there was a range in the measured ME value of pistachios from 12·6 to 27·3 kJ/g.
There was no effect of treatment on plasma total cholesterol, HDL-cholesterol or TAG. Plasma LDL-cholesterol level after consumption of the control diet, 1·5 oz/d treatment, and the 3 oz/d treatment was 1313 (sem 22) , 1239 (sem 22) and 1234 (sem 21) mg/l, respectively. Plasma LDL-cholesterol concentration was 6 % lower after the 1·5 and 3 oz/d treatment albeit there was no additional reduction in LDL-cholesterol from the 1·5 to the 3 oz/d treatment.
Discussion
Nuts in general, and pistachios in particular, have been associated with various health benefits. Hypercholesterolaemic adults consuming a diet containing 2–3 oz pistachios daily showed improvement in lipid profiles(Reference Sheridan, Cooper and Erario29). In another study, two servings of pistachios per day resulted in lowering of total cholesterol, LDL-cholesterol, plasma stearoyl-CoA desaturase activity and other biomarkers of CVD(Reference Gebauer, West and Kay8). Pistachios have also been shown to improve blood glucose concentrations, endothelial function and indices of inflammation(Reference Sari, Baltaci and Bagci14). Thus, inclusion of pistachios in the diet has beneficial influences on health, and accurate information about the energy density of pistachios is important for consumers to make educated decisions about incorporation of pistachios into the diet.
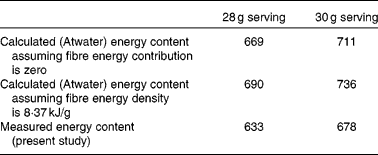
We have measured gross energy of pistachio nuts to be 29·46 kJ/g. The results of this study support our hypothesis that the ME content of pistachios is less than that calculated by the Atwater general factors. The measured ME density of pistachios is 22·6 kJ/g. This value is 5 % less than the currently accepted energy value of 23·7 kJ/g, as calculated using the Atwater general factors (yet neglecting the contribution of fibre, according to current US Food and Drug Administration regulations) and as reflected currently on nutrient labels. Therefore, based on the measured ME of pistachios, 1 oz serving (28 g) provides 633 kJ, and a 30 g serving (as is currently used for many pistachio labels) provides 678 kJ. The current method for calculating energy content based on Atwater general factors results in an energy content of 668·6 kJ for a 28 g serving and 711·3 kJ for a 30 g serving. If the Atwater method was performed with the estimated energy content of fibre being 8·4 kJ/g (the expected energy content of fibre), then the 28 g serving would provide 689·5 kJ and the 30 g serving would provide 736·4 kJ. These values are summarised in Table 5. Labelling regulations permit the assignment of a value of 0 kJ/g for insoluble fibre even though the energy value of insoluble fibre is likely approximately 8·4–12·6 kJ/g. If the Atwater calculation included the energy contribution of fibre, the label value for the energy content of a 30 g serving would be 736–749 kJ.
Table 5 Energy content (kJ) of a serving of pistachios determined by different methods
There was a notable inter-individual variability in the ME values of the subjects studied. There are several factors that likely contribute to the variability including individual uniqueness of the microbial population of the gastrointestinal tract, differences in transit time through the gastrointestinal tract and differences in mastication habits. The degree of mastication has been shown to affect lipid accessibility from nuts(Reference Cassady, Hollis and Fulford30).
Several previous studies have shown that fat from nuts or peanuts is resistant to absorption. As early as 1980, Levine & Silvis(Reference Levine and Silvis24) showed that dietary fat excretion was significantly greater after consumption of whole peanuts compared with peanut butter or peanut oil, and this result was confirmed by Traoret et al. (Reference Traoret, Lokko and Cruz26). Postprandial lipaemia as assessed by plasma TAG was lower after volunteers consumed intact almond seeds compared with almond oil and defatted almond seed starch(Reference Berry, Tydeman and Lewis25). Mechanical disruption of the almond seed by different levels of controlled chewing influenced fat appearance in faeces, with increased mastication resulting in less faecal fat content(Reference Cassady, Hollis and Fulford30).
Ellis et al. (Reference Ellis, Kendall and Ren31) investigated the digestibility of almonds, including the effects of mechanical disruption, chewing and digestion on almond seed microstructure and intracellular lipid release. Inspection of faecal samples from adults consuming almonds revealed intact cotyledon cells (embryonic tissue within the seed of a plant), such that intracellular lipid was encapsulated within the cell walls, protecting it from digestion and absorption. Mechanical disruption (such as chewing) of almond tissue ruptured only cells at the fractured surface layer, leaving cells in deeper layers intact.
The effect on LDL-cholesterol from pistachios added to the diet is consistent with recent published pistachio research and research with other tree nuts. Previously published interventions have resulted in an increase in HDL-cholesterol with tree nut consumption, and the inability to detect an effect on HDL-cholesterol in this study is likely due to the fact that this study was not powered to detect a change in HDL-cholesterol (the effect of pistachios on lipid measurements were conducted opportunistically and not as a primary outcome of this study).
Since this study was conducted in a free-living population, it is possible that subjects might have consumed foods that were not part of the controlled diet. Such non-compliance could add error to the measures. We minimised the probability of non-compliance by observing the consumption of most meals. Nuts were provided at breakfast and dinner so their consumption could be observed as well.
In conclusion, pistachio nuts contain less ME than that calculated from the Atwater general factors. Consumers rely on accurate food labels for making informed dietary choices. Accurate information about ME content of foods is important for reliable food labelling.
Acknowledgements
This work was supported by the US Department of Agriculture, ARS and Paramount Farms, Inc. The authors have no conflicts of interest to report. Author contributions were as follows: D. J. B. and J. A. N. designed the study; D. J. B., J. A. N. and S. K. G. conducted the study and analysed the data and D. J. B. and J. A. N. interpreted data and wrote the manuscript.









