INTRODUCTION
Invasive meningococcal disease (IMD) in Europe for the most part occurs as sporadic cases, with most transmission leading to asymptomatic carriage [Reference Trotter, Gay and Edmunds1]. In several European studies investigating the proportion of subsequent, epidemiologically linked cases, these comprised 3–16% of all cases [Reference Hastings2–Reference Samuelsson6], with higher proportions reported in higher incidence settings [Reference De Wals3, Reference Palau and Noguera5, Reference Samuelsson6]. For instance, in Belgium the proportion of secondary cases (including co-primary cases) was 5·2% in 1971–1973 and 2% in 1974–1976, with IMD incidence decreasing from ~5 to 1 IMD cases/100 000 inhabitants during 1971–1976 [Reference De Wals3]. In Frediksborg county, Denmark, the proportion of secondary cases was 5–16% from 1987 to 1989, during which the overall incidence was extremely high at between 9·7 and 14·1 [Reference Samuelsson6]. While observational studies have shown that chemoprophylaxis of household contacts of persons with IMD to eradicate nasopharyngeal carriage of Neisseria meningitidis reduces the risk of subsequent cases in those contacts [Reference Purcell7, Reference Stefanoff8], this has not been shown for contacts in other settings, although there are numerous reports on the occurrence of secondary cases in pre-school [Reference Hastings2–Reference Palau and Noguera5, Reference Bernabeu9–Reference Samuelsson, Gustavsen and Rønne17], school [Reference Hastings2–Reference Palau and Noguera5, Reference Davison11, Reference Samuelsson, Gustavsen and Rønne17–Reference Zangwill30], and university or college settings [Reference Hastings2, Reference Barker31–Reference Bruce35]. Asymptomatic transmission of the index strain has also been shown in these settings, although to a lesser extent than in household settings [Reference Cartwright, Hunt and Fox10, Reference Sáez-Nieto16, Reference Kristiansen, Tveten and Jenkins36, Reference Wall37]. A survey of 12 European countries performed in 2006 revealed variation in policy regarding chemoprophylaxis for contacts between countries as well as within countries over time [Reference Boccia38]. This was corroborated by unpublished data from a recent survey [Reference Hoek39], in which 12/28 European countries did not recommend chemoprophylaxis for pre-school/day-care contacts (referred to as pre-school from hereon in) of a case of IMD while 16 did. Of these, three recommended chemoprophylaxis for the entire institution and 10 for contacts in the group or class of the index patient (M. Hoek, personal communication). The lack of a common approach to policy development in this area was in part attributed to uncertainty around the effectiveness of preventive measures [Reference Hoek39]. Therefore, the European Centre for Disease Prevention and Control (ECDC) launched the development of guidance on the public health management of sporadic cases of meningococcal disease. This involved systematic literature reviews and the application of GRADE methodology [Reference Atkins40–Reference Schünemann43] to grade the evidence and strength of recommendations [44]. In this paper, we present the results of a systematic literature search performed as part of this project to search for direct and indirect evidence on the effectiveness of chemoprophylaxis in educational settings.
METHODS
We performed a systematic literature review looking for direct and indirect evidence for the effectiveness of chemoprophylaxis in pre-school, school and college contacts of primary IMD cases. As a similar search in 2004 had not found any direct evidence [Reference Purcell7], we presumed the focus would lie on indirect evidence. Therefore, we specifically searched for data permitting a comparison of the incidence of IMD in contacts of primary cases in educational settings with the background risk of sporadic cases. To enable an explicit comparison with risk in household settings, for which there is direct evidence for the effectiveness of chemoprophylaxis of close contacts [Reference Purcell7], we also searched for analogous data for household contacts of sporadic IMD cases.
Search strategy
We searched the literature up to December 2009 in Medline (from 1960), EMBASE (from 1974), Global health (from 1972), Cochrane database of systematic reviews and the Cochrane central register of controlled trials through the German Institute of Medical Documentation and Information (DIMDI, http://www.dimdi.de/static/en/index.html). The following search string was used to retrieve relevant papers: (meningoc? OR neisseria meningit?) AND (chemoprev? OR ?prophyla? OR antibiotic?) AND (transmission OR contact? OR second? OR attack OR cluster? OR outbreak?) AND (?school? OR day care OR nurser? OR child care OR college? OR universit? OR dormitor?), yielding 310 abstracts. To search for further, indirect evidence as described above, we dropped the term (chemoprev? OR ?prophyla? OR antibiotic?) and added the term AND (incidence or risk) to identify any studies investigating subsequent cases of meningococcal disease in contacts of primary cases that would also allow comparison with the background incidence of IMD. This yielded 386 additional papers.
The following search string was used to identify studies comparing the incidence of subsequent cases in contacts in household settings with background incidence: (meningoc? OR neisseria meningit?) AND (transmission OR contact? OR second? OR attack OR cluster? OR outbreak?) AND (household or family). This yielded an additional 238 papers, for a total of 934 retrieved references that were screened.
Inclusion and exclusion criteria
Papers in any European language were accepted. We individually assessed abstracts for relevance to the question and reviewed full papers on relevant abstracts. Papers were selected for inclusion in the evidence assessment if they described analytical or observational studies with comparison groups or studies permitting comparison of incidence in contacts of at least 10 cases in the defined settings with the background incidence of sporadic IMD cases. We examined reference lists in pertinent papers for other relevant publications, and searched Google Scholar for citations of identified key papers.
Identification of unpublished data
To minimize bias, epidemiologists and microbiologists with expertise in meningococcal disease across Europe were identified through established Meningococcal Networks (Meningococcal Disease Society, Invasive Bacterial Infections Network based at ECDC) and asked for unpublished data that fitted our criteria. However, no further studies were identified.
Comparison of IMD risk in contacts of primary cases with background incidence of sporadic cases
In identified observational studies that provided information on subsequent cases of IMD in contacts of index cases of IMD in pre-school, school, college or household settings (see Appendix Table 1, available online) we extracted or calculated the incidence of subsequent cases in 1–30 days (or a period as close to this as possible) after contact to the index case (subsequent attack rate, SAR) in the retrieved studies for household and educational settings. This was then compared to the background age-specific incidence of sporadic cases (ISC) in the same time interval by calculating the relative risk (RR) and risk difference (RD). The required parameters were calculated as follows:


Taylor series 95% confidence intervals (CIs) for RR were calculated using Epi-Info v. 6.04 and CIs for RD were calculated based on the robust approximation of Miettinen & Nurminen [Reference Miettinen and Nurminen45] using an online calculator provided by the University of Manchester (http://www.phsim.man.ac.uk/risk/). Pooled risk estimates were calculated using the metan command in Stata version 11.0 licensed to the Robert Koch-Institute, including an analysis of heterogeneity between studies using the χ2 test with a random-effects model and weighting based on inverse variance.
RESULTS
Search results
Results of our search are summarized in Figure 1.
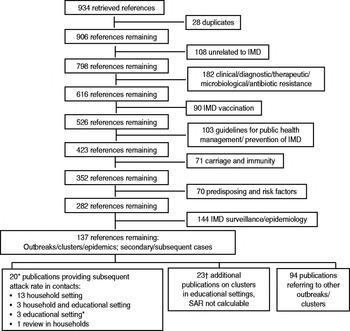
Fig. 1. Flowchart of search results. * One additional publication found in references; † two additional publications found in references. IMD, Invasive meningococcal disease; SAR, subsequent attack rate.
Direct evidence
Our search did not identify any direct evidence, i.e. studies that compared the incidence of subsequent cases in contacts given and not given chemoprophylaxis in educational settings.
Indirect evidence
A retrospective ecological study involving 12 European countries was identified in which countries with a policy of giving chemoprophylaxis only to close contacts after a single case of IMD in a pre-school setting had 3·8 (95% CI 0·7–22·0) times the risk of clusters than countries with a policy of giving chemoprophylaxis to all children in the nursery [Reference Boccia38]. There was a lack of accurate national statistics on the size and number of nursery schools. Co-primary cases were not excluded.
Our search strategy further identified seven observational studies that permitted comparison of IMD incidence in contacts of primary cases in educational settings with background incidence of sporadic IMD cases [Reference Hastings2–Reference Olivares and Hubert4, Reference Davison11, Reference Jacobson24, Reference Zangwill30, Reference Favorova, Sokova and Chernyshova46] (Appendix Table 1). A total of 15 observational studies (16 publications) [Reference Hastings2–Reference Olivares and Hubert4, Reference Davison11, Reference Jacobson24, Reference Zangwill30, Reference Favorova, Sokova and Chernyshova46–59] was identified with information on risk of subsequent IMD in household contacts of sporadic cases, of which only five contained all numerator and denominator data required for a comparison of risk in contacts who had not received chemoprophylaxis with the background incidence of sporadic cases (Appendix Table 5).
Risk of subsequent cases of IMD in contacts of primary cases compared to background risk of sporadic IMD
Pre-school setting
Five studies permitted estimation of risk in pre-school settings [Reference Hastings2–Reference Olivares and Hubert4, Reference Davison11, Reference Favorova, Sokova and Chernyshova46]. In two studies in which chemoprophylaxis was recommended by public health authorities for contacts in the same pre-school as the IMD case, no subsequent cases were observed [Reference Hastings2, Reference Olivares and Hubert4]. In the three other studies where chemoprophylaxis was not generally recommended [Reference De Wals3, Reference Davison11, Reference Favorova, Sokova and Chernyshova46], the risk of subsequent cases in contacts was significantly higher than the background IMD incidence (Appendix Table 2). The pooled estimates of RR and RD from these studies, which fulfilled criteria for homogeneity, were 22·3 (95% CI 12·1–40·9) and 58·2/105 (95% CI 27·3–89·0), respectively (Table 1).
Table 1. Pooled estimates of the relative risk (RR) and risk difference (RD) of incidence of subsequent invasive meningococcal disease (IMD) cases in contacts at ~1 month after contact with a case of IMD and background IMD incidence (detailed data in online Appendix Tables 1–5)
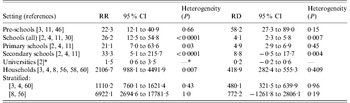
* One study only.
School setting
Five studies permitted calculation of SAR, RR and RD in various school settings (Appendix Table 3). Chemoprophylaxis was not recommended for school contacts in these settings, with the exception of close contacts among classmates in France [Reference Olivares and Hubert4]. SAR was generally lower than in pre-school settings with the exception of the Brazilian study [Reference Oppermann26] (which was, however, undertaken in a very high incidence setting and therefore excluded from the pooled analysis) and the French study [Reference Olivares and Hubert4], where SAR estimates in classroom contacts overlapped with estimates in older pre-school children in the UK [Reference Davison11] and Belgium [Reference De Wals3] (Appendix Tables 2 and 3). In the one study that included both pre-school and school settings without chemoprophylaxis [Reference Davison11] RR and RD were markedly lower in school than nursery contacts (Appendix Table 2). The RRs were consistently statistically significantly elevated in all school-based studies, with a wide range that overlapped with RRs in pre-school settings. Because background incidences were lower in school settings, however, the RDs were consistently lower than in pre-school settings, with a pooled estimate of 4·1/105 (95% CI 2·3–5·8) from one US [Reference Zangwill30] and three European studies [Reference Hastings2, Reference Olivares and Hubert4, Reference Davison11], but with significant heterogeneity between these studies (Table 1). When only data from primary-school children were pooled (possible in three studies [Reference Hastings2, Reference Olivares and Hubert4, Reference Davison11]), the RD estimate was 4·9/105 (95% CI 2·9–6·9) and heterogeneity was no longer significant. When only data from secondary-school children from these studies were pooled, heterogeneity remained significant with a RD estimate of 8·8/105 (95% CI –0·046 to 17·7). In school settings, RR and RD were highest when analyses were restricted to contacts in classrooms [Reference Olivares and Hubert4, Reference Jacobson24] (Appendix Table 3).
University setting
Only one study [Reference Davison11] provided data on risk of secondary cases in the university setting (Appendix Table 4). The size of the contact group was very large (>5000) and the SAR was only marginally higher than baseline (RR 1·5, 95% CI 0·6–3·5).
Household setting
For comparison with the educational settings, in the five household studies (Appendix Table 5) the pooled RRs and RDs were 2254 (95% CI 947–5362) and 401/105 (95% CI 263–539), respectively (Table 1). The observed substantial heterogeneity in the RR estimate from these five studies was resolved by stratification, leading to a more conservative estimate of RR of 1110·2 (95% CI 760·1–1621·4) but a slightly higher RD of 480·1 (95% CI 321·5–639·9) when the two studies with extremely high RR (and low number of primary and secondary cases) [Reference Stefanoff8, Reference Samuelsson56] were excluded (Table 1).
Timing and exact setting of subsequent cases within educational institutions
Exact data on the time interval between occurrence of the primary and subsequent cases were not available in all studies. Available data in two studies suggested that about 70% of subsequent cases occurred within 1 week and 90–100% within 3 weeks [Reference De Wals3, Reference Davison11]. In the US study in schools, 33% of subsequent cases occurred within 1–2 days and 73% within 14 days [Reference Zangwill30]. Davison et al. [Reference Davison11] reported that 57% of all subsequent cases occurred in the same grade or class in pre-school and school settings combined, and Zangwill et al. [Reference Zangwill30] reported that 55% of subsequent cases were in a different grade than the index case.
DISCUSSION
Our search did not identify direct evidence for the effectiveness of chemoprophylaxis in contacts of IMD in educational settings as no studies compared the incidence of subsequent cases in treated and untreated contacts. However, we found indirect evidence that permitted a comparison of the risk of subsequent cases in contacts of persons with IMD in educational settings with the background risk in a defined time interval of ~30 days after occurrence of the index case. Because direct evidence exists for the effectiveness of chemoprophylaxis of contacts in household settings, we also calculated the risk of subsequent cases in household settings in contacts who did not receive chemoprophylaxis and compared this to background risk. Our study showed that contacts in pre-school and school settings in which chemoprophylaxis was not generally recommended had a significantly increased risk of IMD, but that this risk was markedly lower than for household contacts.
The studies included in our analysis have a number of limitations. While they applied directly to the populations of interest, background incidences varied. Active prospective follow-up of contacts to ascertain subsequent cases was not performed in all household studies (Appendix Table 1). All key studies in educational settings but one collected at least some of the data retrospectively (Appendix Table 1) and lacked data on potential confounding variables such as socioeconomic factors and other risk factors for IMD. Some studies included co-primary cases [Reference Hastings2, Reference Davison11], which would lead to an overestimation of SAR and RR. In addition, the studies varied in the definition of the time interval for ascertainment of subsequent cases, which ranged from 28 days to 4 months. However, as the risk of subsequent cases approaches the background risk after 3–4 weeks, the RR and RD would tend to be lower by inclusion of studies with a longer observation period. In all studies, the number of contacts of primary cases was estimated based on available national data on mean group or class size and size of institutions and thus probably diverged from the true situation in the few outbreaks that occurred. Furthermore, calculation of SAR was often based on very small numbers of subsequent cases (sparse data), and the observed increased risk of IMD in contacts vs. the background risk tended to be higher in less precise studies with smaller numbers of cases. Moreover, recognition of a case among contacts may be more likely than in the general population, thus background incidence may be underestimated and RR overestimated. However, as IMD is such a severe disease, under-ascertainment of primary cases is likely to be lower than for many other infections. Data were lacking on whether any contacts actually obtained chemoprophylaxis; information was only given as to whether chemoprophylaxis was generally recommended or not in the respective setting when the study was undertaken. Receipt of antibiotics by contacts would lead to falsely low estimates of SAR. Only three studies [Reference Hastings2, Reference Davison11, Reference Zangwill30] provided data on strain characterization of at least a proportion of primary and subsequent cases; these suggested that meningococcal strains are identical in primary and subsequent cases in most instances.
In view of these limitations it is, nonetheless, remarkable that the evaluated studies consistently showed a significantly increased risk for the occurrence of subsequent IMD cases compared to the background incidence of primary cases in a time interval from 0–2 to 28–120 days after illness onset in the index case in pre-school and school settings where chemoprophylaxis was not generally recommended. Compared to background incidence, the risk of acquiring IMD was significantly elevated in pre-school settings (RD 58·2/105, 95% CI 27·3–89·0) when chemoprophylaxis was not recommended. This is underscored by the absence of subsequent cases in pre-school contacts in settings with clear recommendations for chemoprophylaxis in place [Reference Hastings2, Reference Olivares and Hubert4]. In fact, in England and Wales, no subsequent cases of IMD were observed in pre-school settings from 1992 to 1995, when chemoprophylaxis was recommended, but were observed starting in 1995–1996, when this recommendation was rescinded. While clusters due to serogroup C did not occur after 1999–2000, when mass vaccination against IMD due to serogroup C took place in the UK [Reference Trotter and Ramsay61], serogroup B clusters continued to be observed in educational settings [Reference Granerod12, 62]. Our findings are also supported by an analysis of IMD clusters in Barcelona from May 1995 to December 1997 [Reference Palau and Noguera5], in which seven of the 13 observed clusters occurred in nurseries [two occurred in schools, one in siblings (family members received chemoprophylaxis in 93·6% of cases), two in military settings and one in members of the same village]. Our estimated RD for pre-schools was an order of magnitude lower than that for household settings (401/105, 95% CI 263–539), where chemoprophylaxis has been estimated to decrease the risk of subsequent cases in contacts by ~86% [44]. Under the assumption that chemoprophylaxis would be similarly effective in pre-school settings, the number of contacts to be treated (NNT) with an antibiotic to prevent one case can be estimated as RD 1/0·86. Based on the above data, this yields an estimate of 1998 (95% CI 1307–4259) children, compared to 290 (95% CI 216–442) in household settings. Thus the cost of preventing one case would be markedly higher than in households. Furthermore, in educational settings it may take longer or be more complex to arrange for all contacts to receive antibiotics, thus the effectiveness of chemoprophylaxis may well be lower than in households.
While RR estimates overlapped, RD estimates in school settings were an order of magnitude lower than in pre-school settings, largely because the background risk in older children was lower than in younger children. Thus, NNT estimates would be markedly higher still. The study in university settings was limited by the large size of the contact group, which may have made recognition of epidemiologically linked cases more difficult; defining smaller contact groups might have led to higher estimates of SAR.
None of the studies included in the meta-analyses mentioned educational workers. A review article on the risk of educational workers [Reference De Wals63] listed only one reference that identified teachers who contracted IMD. In this study, which reviewed 50 adult IMD cases ascertained from 1997 to 1999 in Cheshire, UK, seven cases were identified in educational workers with a sixfold higher risk compared to the general adult working population [Reference Woodhouse and Hunter64]. The authors recommended caution in interpretation of these results due to the small area investigated during a period of increased incidence; however, the results suggest that school workers should be included in the risk assessment of contacts of IMD cases.
While a review of 18 studies did not find any reports of severe adverse events in conjunction with antimicrobial chemoprophylaxis with rifampicin, cipropfloxacin, ceftriaxone or azithromyicin, mild side-effects of recommended antibiotic regimens were common [Reference Fraser65]. A case of anaphylaxis and two anaphylactoid reactions have been described in conjunction with chemoprophylaxis using ciprofloxacin [Reference Fraser65–Reference Giovanetti67]. Thus severe side-effects may rarely occur, particularly if the number of persons defined as contacts is large. The development of resistance has been observed in randomized controlled eradication trials with rifampicin, with 10–27% of initial carriers developing resistance in three randomized controlled trials [Reference Blakebrough and Gilles68–Reference Taha71]. Emergence and spread of rifampicin resistance has also been observed in several non-controlled studies [Reference Jackson22, Reference Cooke48, Reference Devine72, Reference Lepe73] and cases due to rifampicin-resistant meningococcal isolates have also been reported after prophylaxis [Reference Cooke48, Reference Almog74–Reference Rainbow77]. On the other hand, resistant N. meningitidis has not spread widely, possibly because the acquisition of rifampicin resistance appears to confer a biological disadvantage [Reference Taha71]. Ciprofloxacin-resistant N. meningitidis was isolated in the USA in 2009 from three IMD cases and two carriers, one after contact with one of the patients and after receipt of ciprofloxacin [Reference Wu78]; no further cases have been described after recommendations to use other antibiotics for prophylaxis in the affected regions. Another theoretical negative effect of chemoprophylaxis is the eradication of N. lactamica from the nasopharynx. Colonization of N. lactamica is associated with the induction of cross-protective immunity to N. meningitides [Reference Gold79, Reference Coen, Cartwright and Stuart80]. Carriage of N. lactamica is highest in nursery-aged children [Reference Gold79, Reference Bakir81] and prior antibiotic therapy has been shown to decrease carriage [Reference Bakir81].
Because IMD is associated with a high risk of complications and death its occurrence generates substantial anxiety in contacts [Reference Taylor-Robinson82]. Thus we believe that contacts would want chemoprophylaxis even if evidence for benefit is weak, as direct harmful effects are rare and further risks largely theoretical. This is in keeping with comments from The Meningitis Trust, a non-governmental organization in the UK with a public helpline, that it is difficult to convince parents of children attending the same nursery/playgroup as a case that prophylaxis is not needed.
As described in detail elsewhere [44], GRADE methodology was applied to the results of this review after taking into account the above limitations and after weighing the potential benefits and harms in light of disease severity and risk in the settings. This resulted in the recommendation that attending the same pre-school as an IMD case should be considered an indication for chemoprophylaxis, depending on risk assessment. The risk assessment should take into account duration and closeness of contact, as the risk of further cases is likely to be higher in settings similar to households, where risk of exposure to respiratory droplets would be more likely. Some studies found a higher risk for IMD in more crowded household settings [Reference Kaiser51, Reference Baker83–Reference McCall, Neill and Young85] and crowded conditions were described in several day-care-associated outbreaks in the USA [Reference Jacobson, Filice and Holloway13, Reference Leggiadro14]. Thus children in the same group as the index case who have spent long periods in the same room (e.g. full-time attendance, sharing meals, napping together) are likely to be at higher risk than children in a different group.
The results of this systematic review suggest that attending the same school/college (including the same class) as a first case of IMD should not in itself be considered an indication for widespread chemoprophylaxis in contacts. Here also, the decision for whom chemoprophylaxis is indicated should be based on an assessment of whether household-like contact may have occurred among school friends, e.g. spending nights together. In two school outbreaks various extracurricular activities or excursions were identified as possible settings in which transmission may have occurred [Reference Morrow25, Reference Robinson27].
Regardless of whether they receive chemoprophylaxis, all contacts should be informed of early symptoms of disease and the importance of seeking immediate medical advice, as failure to do so has been described and re-introduction of the index strain cannot be ruled out due to the large number of social contacts involved in educational settings. Indirect evidence is available suggesting that vaccination of close contacts of index cases with IMD due to a vaccine-preventable strain prevents late secondary cases [Reference Hoek86], and thus post-exposure vaccination is strongly recommended in the ECDC guidance document [44]. Further prospective studies on the risk of subsequent cases and the transmission of disease-causing strains in educational settings are needed. Prospective studies on the risk of subsequent cases in contacts who receive and do not receive chemoprophylaxis may be feasible as an initiative involving several countries with divergent public health policies.
In conclusion, we found limited but consistent evidence that the risk of IMD in pre-school contacts of sporadic cases is significantly increased above the background risk in European settings. In addition, in two studies performed in settings with a recommendation for prophylactic treatment of pre-school contacts, no subsequent cases were observed. This is in keeping with results of the retrospective ecological study performed by Boccia et al. [Reference Boccia38], which suggested a lower risk of IMD clusters in countries in which prophylaxis of pre-school contacts was recommended compared to those without such a recommendation, although the difference was not statistically significant. Although direct evidence for the effectiveness of chemoprophylaxis has only been shown in household settings, where the risk of subsequent cases is an order of magnitude higher, we recommend chemoprophylaxis for pre-school contacts based on an assessment of duration and closeness of contact. In school or college settings chemoprophylaxis should be offered only when there is evidence of close prolonged contact with the index case.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
ACKNOWLEDGEMENTS
This study was funded in part by the European Center for Disease Prevention and Control (ECDC). We thank Pierluigi Lopalco and Helena de Carvalho Gomes from ECDC for technical and expert advice. We thank Ole Wichmann for helpful comments on the manuscript.
DECLARATION OF INTEREST
None.






