- COX-2
cyclo-oxygenase 2
Associations between human dietary patterns and risk of chronic disease are well documented. Numerous epidemiological and laboratory studies have suggested protective effects of a variety of nutritionally essential plant-based dietary components, such as fibre, antioxidant vitamins and trace elements. However, plants also contain more than 100 000 secondary metabolites ranging from structurally simple alkaloids to more complex phytosterols and polyphenolic molecules(Reference Dillard and German1). These ‘phytochemicals’ are not recognised as essential dietary components as a lack is not associated with a specific deficiency condition. Nevertheless, many of these non-nutritive compounds exert biological activities in mammalian systems that may impact on health and disease risk. Indeed, some have been used therapeutically since ancient times and their molecular structures are the basis of many modern pharmaceuticals. As such, bioactive compounds are likely to be present in a wide range of plant-based foods and there is growing interest in their potential role in health and disease prevention in nutritionally relevant amounts. Several recent reviews discuss the potential importance to health of dietary flavonoids, terpenoids, phyto-oestrogens and glucosinolates(Reference Spencer2–Reference Johnston5). To date, less attention has been focused on phenolic acids. These simple molecules are not only widespread in plant foods but are also the synthetic precursors and catabolic products of many more complex phytochemicals. There is growing interest in the possibility that some phenolic acids, such as salicylic acid, may prevent the development of gut disorders such as colon cancer. In this context, we consider whether phenolic acids of plant origin may benefit gut health.
What are plant secondary metabolites?
All living cells possess similar pathways for the synthesis of components such as sugars, amino acids, bases, carbohydrates, proteins, nucleotides and these are essential for primary metabolism. Plant secondary metabolites are derived from the products of primary metabolism but have a much more limited taxonomic distribution. Some are produced for appreciable reasons (e.g. defence, colourants and attractants), but for many their functions and benefits to the plant are essentially unknown. They can be broadly categorised according to their structure and biosynthetic pathways (Fig. 1). However, it should be appreciated that many secondary metabolites are derived by combining elements from all these biosynthetic routes.

Fig. 1. Main biosynthetic routes in plants from which plant secondary metabolites are derived. Many secondary metabolites are derived by combining elements from all these biosynthetic routes. hv, energy from sunlight; TCA, tricarboxylic acid cycle.
Nitrogen- and sulphur-containing compounds
Alkaloids contain one or more amino acid-derived nitrogen atoms and are structurally the most diverse class of secondary metabolites. Their production is commonly associated with allelopathic effects in host plant defence and signalling. They have a wide range of pharmacological activities and a historical use as stimulants, medicines and toxins. For example, hemlock (Conium macuatum) was a mainstay of the poisoners’ repertoire as it contains coniine, an alkaloid that paralyses motor neurone nerves(Reference Croteau, Kutchen, Lewis, Buchanan, Gruissem and Jones6). Many drugs in modern use contain alkaloids or their synthetic analogues. Examples are atropine, codeine, heroin, morphine, cocaine and vinblastine. From a dietary perspective, alkaloids such as capsaicin are responsible for the fiery taste of chilli peppers and the purine alkaloid, caffeine, causes the stimulatory effects associated with drinking a cup of coffee(Reference Benowitz7).
Glucosinolates contain both S and N. Their function in plants is unclear, but a likely role is to discourage herbivory, as they produce enzymically hydrolysed toxic metabolites in damaged plants. These products also provide the intense pungent flavour observed in the Brassicaceae vegetables (e.g. mustard and horse radish). Broccoli and Brussels sprouts are rich dietary sources, but are not to everyone's taste. Glucosinolates have limited biological activity but the glucose moiety is removed by myrosinase released from cell membranes by chewing or processing. The resulting aglycone can form compounds such as isothiocyanates and indoles. These have been extensively studied in relation to protection against carcinogenesis and mutagenesis(Reference Hayes, Kelleher and Eggleston8–Reference Mithen, Dekker and Verkerk10).
Secondary metabolites of the acetate pathway
The acetate pathway produces a wide range of natural products including the fatty acids and polyketides. The formation of the poly-β-keto chain results from carboxylation of acetyl-CoA to malonyl-CoA and a series of Claisen condensation reactions. Plants contain predominantly unsaturated fatty acids with the dietary supplement γ-linolenic acid being derived from Borage (Borago officinalis) and Evening Primrose (Oenthera biennis). The growing poly-β-keto chain can be stabilised by cyclisation and further reaction. The resulting bioactive polyketides have a range of applications. These include use as stimulant laxatives, antibiotics and antifungals(Reference Herbert11).
Secondary metabolites of the mevalonate pathway
Terpenoids, often referred to as terpenes or isoprenoids, are a structurally diverse group of hydrocarbons derived from the five-carbon precursors: isopentyl diphosphate or dimethylallyl diphosphate. Over 25 000 terpenoids have been identified(Reference Croteau, Kutchen, Lewis, Buchanan, Gruissem and Jones6) and they are classified according to the degree of isoprene incorporation, i.e. hemiterpenes (C5), monoterpenes (C5)2, sesquiterpenes (C5)3, diterpenes (C5)4, sesterpenes (C5)5, triterpenes (C5)6 through to higher polymers such as rubber (>100). In addition to being biosynthesised as part of plants’ host defence, flowers emit terpenes to attract pollinating insects(Reference Maimone and Baran12). Bioactive molecules derived from terpenoids include the herbal tranquilliser, valtrate, the principle component of valerian (Valeriana officinalis) and the anti-cancer drug, taxol, extracted originally from the Pacific Yew (Taxus brevifolia). The tetraterpenes (C5)8, collectively known as the carotenoids, play a major role in photosynthesis. They are highly coloured pigments. For example, lycopene imparts the characteristic red colour to tomatoes and β-carotene the orange colour in carrots. Higher modified terpenoids include the phytosterols, steroidal saponins such as diosgenin in Fenugreek (Trigonella foenum-graecum) and the cardioactive glycoside, digitoxin, from Foxglove (Digitalis purpures). Additional major food sources include citrus fruits, apricots, soya, grapes, grains, spinach, kale and sweet potatoes. Numerous effects of terpenoids in mammalian cells have been reported including anti-tumour and antioxidant activity although whether such affects are achievable at nutritionally relevant intakes is not always clear. Several reviews consider the possible relevance of terpenoids to health and disease prevention(Reference Tirapelli, Ambrosio and de Oliveira13–Reference Rao and Rao15).
Secondary metabolites derived from the shikimate pathway
Approximately 20% of carbon fixed by plants is channelled into the shikimate pathway. The plant metabolites produced by this primary pathway are an essential component of our diet. The shikimate pathway has been described as ‘a metabolic tree with many branches’(Reference Bentley16) although chlorisimate formation is generally considered as the major branch point. Beyond chlorisimate the pathway provides a synthetic route to folate coenzymes, enterobactins, siderophores, lipid-soluble isoprenoid quinones and the three essential aromatic l-amino acids, tryptophan, tyrosine and phenylalanine.
As well as performing a significant role in the provision of primary metabolites, the shikimate pathway has a major function in higher plants in the production of secondary metabolites(Reference Bentley16). The switch from primary to secondary metabolism occurs with the E2-elimination of ammonia from the amino acid, l-phenylalanine, to form cinnamic acid, the first metabolite of the phenylpropanoid pathway. The C6C3 structure of cinnamic acid is an essential building block for the largest range of natural products suggested as potentially beneficial for human health. Such compounds are characterised by having at least one aromatic ring with one or more hydroxyl groups and range from complex structures with high molecular weight such as the plant polymer lignin to simple, single-aromatic compounds. Such compounds have diverse functions in plants including acting as antioxidants and signalling molecules, providing skeletal structure, aiding pollination and protecting against microbial infection, herbivorous grazing and excessive UV light(Reference Haslam17).
Phenolic acids
Broadly speaking, simple phenolic acids in plants are derived from an ortho oxygenation and subsequent methylation substitution of cinnamic acid. This gives rise to the most common parent hydroxycinnamic acids, namely: p-coumaric acid, caffeic acid, ferulic acid and sinapic acid (Fig. 2). These are often considered as intermediates of lignin biosynthesis(Reference Russell, Burkitt and Provan18). However, they are also the important building blocks of many other natural products and are often found as specific esters and dehydrodimers (e.g. chlorogenic acid, truxillic and truxinic acid)(Reference Ralph, Quideau and Grabber19, Reference Russell, Hanley and Burkitt20).
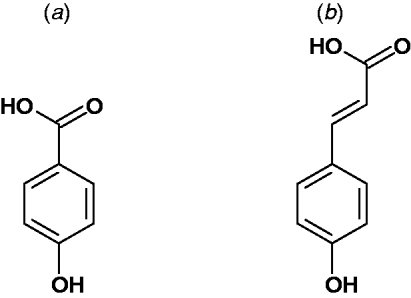
Fig. 2. Parent structures of the predominant phenolic acids found in plants: (a) Hydroxybenzoic acid and (b) hydroxycinnamic acid. Further substitution of the aromatic ring provides the common hydroxybenzoic acids; salicylic acid, protocatechuic acid, gallic acid, syringic acid, vanillic acid and gentisic acid. Examples of hydroxycinnamic acids are p-coumaric acid, ferulic acid and sinapic acid. They are found in plants in their free form, as dimers and conjugated to other plant components such as simple sugars, organic acids and plant polymers.
The C6C1-substituted hydroxybenzoic acids can be formed directly from intermediates early in the shikimate pathway. However, in plants they are more frequently formed by degradation of C6C3 cinnamic acid derivatives. Commonly found examples are 4-hydroxybenzoic acid, protocatechuic acid, vanillic acid and syringic acid (Fig. 2). Less abundant are hydroxyphenylacetic acids (the C6C2 derivatives). Generally, they are observed to have the same substitution pattern as observed for the hydroxybenzoic and hydroxycinnamic acids, but the direct route to their biosynthesis is unclear. Phenolic acids can be found in plants not only in their free form but also conjugated (predominantly by esterification) to a variety of molecules including simple sugars, organic acids and plant polymers.
Dietary sources of phenolic acids
Food composition databases for macro- and micronutrients provide essential information for research on the health effects of nutrients, nutritional surveillance, clinical dietetic practice and food formulation and processing. However, analogous compositional information on potentially bioactive phytochemicals, including phenolic acids, in foods is generally lacking. Several databases of some plant secondary metabolites in commonly consumed foods are under construction(Reference Pérez-Jimenz, Neveu and Vos21–Reference Brat, Georgé and Bellamy23), but generally there is a marked disparity in the literature even for similar food items. This can be ascribed, in part, to differences in the analytical methodology employed between studies. Many employ redox colorimetric assays that show little specificity(Reference Everette, Bryant and Green24). Others measure only those compounds that are easily extracted into solvents. This is likely to result in a marked underestimate of the phenolic acid content as many, in particular the hydroxycinnamic acids, are esterified to insoluble plant fibres. In addition, the phytochemical content of primary food products is also influenced by numerous other factors including plant varieties, seasonality, growing conditions, storage and cooking(Reference Cermak, Durazzo and Kammerer25–Reference Lempereur, Rouau and Abecassis27). Thus, estimating dietary intakes of phenolic acids becomes distinctly problematical and highlights the importance of regular quantitative analysis of food products used during human dietary interventions.
With these caveats, copious literature of which a selection is cited(Reference Talcott, Passeretti and Duncan28–Reference Gil, Tomas-Barberan and Hess-Pierce40) indicates that phenolic acids are ubiquitously distributed throughout plant primary products. Rich sources of hydroxycinnamic acids such as ferulic, sinapic and caffeic acids include legumes, cocoa, fruits, oils, herbs, spices, nuts, vegetables and cereals. In addition, beverages such as coffee, beer and wine are important dietary sources. Some foods are particularly rich in particular hydroxycinnamates. For example, coffee has been reported to contain 1·13 mg caffeic acid/100 ml and green olives have 83 mg sinapic acid/100 g. The hydroxybenzoic acids generally are found in lower concentrations in plant-based foods compared with hydroxycinnamic acids. Significant dietary sources include beverages (fruit juice, tea, beer, wine and spirits), berries, herbs and spices. Up to 14 mg vanillic acid/100 g basil has been reported. Walnuts are rich in syringic acid (57·45 mg/100 g) and 100 g tea can contain up to 10·35 mg gallic acid. Soft fruits appear to be particularly rich dietary sources of both free and esterified hydroxybenzoic and hydroxycinnamic acids compared with other commonly consumed fruit(Reference Russell, Scobbie and Labat41) (Fig. 3).

Fig. 3. Comparison of cinnamic (C6C3) and benzoic (C6C1) acids in commonly consumed and locally produced Scottish soft fruits compared with supermarket-purchased imported fruit. Values are specified on a wet-weight basis in mg/100 g, which corresponds to approximately one fruit portion and are given as mean (sd) (n 3). Adapted from Russell et al. (Reference Russell, Scobbie and Labat41).
Consequently, daily intakes of phenolic acids are likely to be in the milligram range and comparable with many essential micronutrients. For example, estimated mean intakes (mg/d) of some phenolic acids in Finnish adults are: caffeic, 417; ferulic, 129, gallic, 33; p-coumeric, 16; sinapic, 11(Reference Ovaskainen, Törrönen and Koponen42). Estimated intakes in a Bavarian population of protocatechuic acid, vanillic acid and syringic acid are 1·69, 4·17 and 4·48 mg/d, respectively(Reference Radtke, Linseisen and Wolfram43). However, such values may be an underestimate as the composition databases used to calculate daily intakes may not fully consider conjugated forms of the phenolic acids(Reference Tomās-Barberān and Clifford44).
Gut microbiota as sources of phenolic acids
Many of the diverse species of bacteria that constitute the gut microbiome can perform reactions that transform complex plant phenolics such as anthocyanins, procyanidins, flavanones, flavonols, tannins and isoflavones into simple phenolic metabolites. Several Bacteroides, Streptococcus and Clostridium species have been observed in culture to metabolise quercetin, kaempferol, naringenin, diadzein and catechins(Reference Atkinson, Frankenfeld and Lampe45–Reference Selma, Espin and Tomās-Barberān47). Phenolic acids have also been shown to be transformed by the gut microbiota to metabolites some of which retain the phenolic acid structure. For example, once ferulic acid is released from plant cell wall components by the gut microflora it can then be further metabolised undergoing hydrogenation of the α,β-unsaturated bond, demethylation and selective dehydroxylation at C4 to form a plethora of related phenolic metabolites(Reference Russell, Labat and Scobbie48). However, the gut microbiome varies greatly between individuals and so, in vivo, it is difficult to fully ascertain the parent compounds from which phenolic metabolites are derived. In addition, such phenolic acids may modulate the gut microbial population. Gallic acid and caffeic acid are reported to repress Clostridiium and Bacteroides species(Reference Lee, Jenner and Lowa49). As yet, such phenolic–microbiota interactions are not well understood but it is likely that they may influence the degradation and transformation pathways of more complex phenolic compounds. Despite such complexity, it is reasonable to assume that the colon is a rich source of potentially active phenolic acids, concentrations reaching the mm range for some molecules(Reference Jenner, Rafter and Halliwell50). These may impact locally as well as systemically on gut health.
Potential biological activity of phenolic acids
There is increasing evidence that alterations in inflammatory pathways are a key step in the development of gut disorders including colon cancer(Reference Terzić, Grivennikov and Karin51). Consequently, one obvious molecular target for phenolic acids in maintaining gut health is cyclo-oxygenase 2 (COX-2) as this enzyme is strongly and rapidly induced in response to mediators of inflammation, growth factors, cytokines and endotoxins. In cytokine-stimulated human colon fibroblasts, several phenolic acids decrease the production of potentially neoplastic PG that arise from COX-2-mediated catalysis of arachidonic acid(Reference Russell, Scobbie and Chesson52) (Fig. 4). In general, such compounds tend to bind strongly with the COX-2 binding site. In contrast, others such as gallic acid appear to be markedly pro-inflammatory in this model system, possibly by affecting those signalling pathways leading to the upregulation of COX-2 (Fig. 4). Studies using cell and animal models show the effects of phenolic acids on both the expression and activity of enzymes involved in the production of inflammatory mediators(Reference Larrosa, Luceri and Vivoli53–Reference Na, Lee and Oh62). For example, COX-2 expression and activity are reduced by p-coumaric acid in dextran/sodium sulphate (DSS)-induced inflammation in a rodent model(Reference Ye, Liu and Henderson57) and caffeic acid suppresses the expression of IL-17 and ameliorates DSS-induced colitis in mice(Reference Luceri, Guglielmi and Lodovici56). Clearly, these compounds have anti-inflammatory properties in model systems but results have to be interpreted with caution in a nutritional context. In such studies doses often markedly exceed that which may be achievable from the diet. Whether analogous effects occur at dietary relevant concentrations is often unclear. Although some observational studies(Reference Nanri, Yoshida and Yamaji63, Reference Holt, Steffen and Moran64) suggest inverse associations between the consumption of phenolic-rich diets and inflammatory markers, in general, the impact of dietary phenolic acids on gut health in human subjects has not yet been assessed in adequately powered and controlled dietary intervention trials.

Fig. 4. Potential pro- and anti-inflammatory activity of dietary phenolic acids assessed by the ability to modulate the production of prostanoids in cytokine-stimulated human colonic fibroblasts. Adapted from Russell et al. (Reference Russell, Scobbie and Chesson52).
Many potentially beneficial effects of phenolic acids interpolated from in vitro studies may not be of nutritional relevance unless phenolic acids gain access in vivo to appropriate cellular sites such as the colonocytes lining the gut. Systemic and colonic bioavailability of phenolic acids is not well understood and the complexity of absorption and hepatic and microbial metabolism make the correlation of dietary intake with physiological effects distinctly problematical. The exact mechanisms for the absorption of phenolic acids are not clear, but may be passive or involve H+ and Na+ transport systems and/or monocarboxylic acid transporters(Reference Konishi, Zhao and Shimizu65, Reference Konishi and Shimizu66). A recent review(Reference Zhao and Moghadasian67) concludes that the small intestine and colon can both be absorption sites for phenolic acids, with conjugated forms mainly present in the diet generally having a low bioavailability compared with the aglycones. Limited data indicate that relative bioavailability of some hydroxycinnamic acids is chlorogenic<caffeic<ferulic<p-coumaric(Reference Zhao and Moghadasian67). In general, hydroxybenzoic acids appear to be more readily absorbed than hydroxycinnamic acids. Only the benzoic acids were detected in plasma of human volunteers following consumption of strawberries rich in both types of phenolic acids, 26% being recovered in the urine within 5 h of consumption(Reference Russell, Scobbie and Labat68) (Fig. 5). These observations strongly suggest that the bulk of the cinnamic acids escape early absorption in the gastro-intestinal tract. They appear to be destined for the colon where they are released and metabolised by the microbiota. Moreover post-prandial concentrations of phenolic acids in plasma are low (<1 μm) indicative of low levels of absorption and/or rapid hepatic metabolism and excretion(Reference Russell, Scobbie and Labat68). It is likely that any bioactivity that contributes to gut health may predominantly be located in the lower gastro intestinal tract.

Fig. 5. Concentration (ng/cm3) of phenolic acids recovered in plasma after consumption of strawberries rich in hydroxycinnamic acids and hydroxybenzoic acids. Data given as mean (sd) (n 4). Adapted from Russell et al. (Reference Konishi and Shimizu66).
Salicylic acid and colon cancer prevention
In 1763, the Reverend Edward Stone informed the Royal Society that willow bark contained substances that effectively relieved the symptoms of ‘ague’ (malarial fever)(Reference Stone69). The anti-inflammatory and anti-pyretic component was eventually identified as salicylic acid. In Victorian times, large doses were routinely used to treat fever, pain and inflammation but had the unfortunate side effect of causing ulceration of the stomach(Reference MacLagan70). In order to partially address this, an acetylated form was produced (aspirin) at the end of the 19th century. Since then, aspirin has remained the most commonly prescribed drug for relieving pain, inflammatory symptoms and fever(Reference Jeffreys71). More recently, evidence has been accumulating with regard to the fact that regular intake of aspirin inhibits the incidence of, progression, and death due to colorectal cancer. These benefits transcend study designs, and cohort characteristics (e.g. age, gender, nationality, risk factors)(Reference Elwood, Gallagher and Duthie72). Aspirin is deacetylated following consumption. The acetyl group directly and irreversibly binds to the PG H-synthases, inhibiting and modifying the production of neolastic prostanoids. Salicylic acid may contribute to this activity by competitive inhibition of arachidonic acid metabolism and/or some of the other postulated mechanisms of action(Reference Elwood, Gallagher and Duthie72). This has led to the intriguing suggestion that the recognised effects of consuming fruit and vegetables on lowering risk of colon cancer may be due in part to salicylates in plant-based foods(Reference Paterson and Lawrence73). However, it is unclear as to whether sufficient salicylic acid can be obtained from dietary sources to exert disease preventative activity. Estimates of daily intake vary widely ranging from 0·4 to 200 mg/d(Reference Wood, Baxter and Thies74). Using a recently constructed food composition database(Reference Wood, Baxter and Thies74) describing median salicylate values for twenty-seven different types of fruit, twenty-one vegetables, twenty-eight herbs, spices and condiments, two soups, and eleven beverages, estimated median dietary intakes of a Scottish population were 4·4 and 3·2 mg/d for males and females, respectively. Major dietary sources of salicylates were alcoholic beverages (22%), herbs and spices (17%), fruit (16%), non-alcoholic beverages including fruit juice (13%), tomato-based sauces (12%) and vegetables (9%). Intuitively, such salicylate intakes appear insufficient to exert preventative effects and indeed could be negated by the substantial proportion of salicylic acid derived from beverages containing alcohol, a recognised pro-carcinogen. However, serum and urinary salicylate concentrations of vegetarians are higher than omnivores and overlap with individuals who regularly take low-dose aspirin(Reference Blacklock, Lawrence and Wiles75) suggesting substantial absorption of salicylates from ingested plant-based foods. Moreover, populations that incorporate substantial amounts of salicylate-rich spices in foods may have markedly higher daily intakes of salicylates. Indeed, it has been suggested that the low incidence of colorectal cancer among Indian populations may be ascribed in part to high exposure to dietary salicylates throughout life from spice consumption(Reference Paterson, Srivastava and Baxter76). However, plant products found to contain salycilic acid are generally found to be rich in other phenolic acids and the contribution of these compounds to the protective effect should not be overlooked.
Future perspectives
Our gut is exposed to a plethora of simple phenolic acids and related metabolites arising directly from the food we consume and via the microbial degradation of more complex dietary phenolic compounds. Many of these metabolites have potent bioactivities in model systems, which could potentially contribute to gut health. However, at present we remain largely ignorant as to which of these compounds are beneficial (or indeed detrimental). Moreover, understanding of their mechanisms of action in vivo and how they interact with the gut microbiota is likely to help in the prevention of conditions such as inflammatory bowel disease and colon cancer. Future research directions are likely to expand on current metagenomic and metabolomic approaches(Reference Gill, McDougall and Glidewell77–Reference Jacobs, Deltimple and van Velzen79) to elucidate which pro-inflammatory and potentially carcinogenetic changes in gene expression can be moderated by simple phenolic acids. In addition, the development of good methods for monitoring total bacterial communities and their metabolic activity in response to phenolic acids is essential. At present, there is arguably insufficient information to allow the recommendation of specific plant-based foods rich in particular phenolics to optimise gut health. However, in view of the preventative effects of acetylsalicylic acid on the development of colon cancer, more studies on the nutritional efficacy of phenolic acid-rich foods appear warranted.
Acknowledgements
The authors are grateful for funding from the Scottish Government. The authors declare no conflict of interest. The authors contributed equally to the writing of the paper.









