INTRODUCTION
Chikungunya virus (CHIKV), an arbovirus, has caused several epidemics in Africa and Asia [Reference Charrel, de Lamballerie and Raoult1–Reference Thiboutot3]. This genus Alphavirus (family Togaviridae) is transmitted by the Aedes mosquito [Reference Broom, Cook and Zumla4, Reference Pialoux5]. The illness is characterized by acute febrile onset of intense musculoskeletal (MSK) pain and arthralgias. There is no specific treatment but patients tend to be over-treated with analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) [Reference Kamath, Das and Parikh6]. Ten percent of cases allegedly suffer from chronic MSK pain and arthritis [Reference Broom, Cook and Zumla4, Reference Brighton, Porzesky and De La Harpe7]. Although not considered fatal, there is significant morbidity and loss of productive work [Reference Pialoux5]. Community and prospective studies are sparse.
In 2006, India had an unprecedented CHIKV epidemic after 32 years [Reference Lahariya and Pradhan8–Reference Chhabra12]. Although considered an urban problem [Reference Kalantri, Joshi and Riley9, Reference Seligman13], CHIKV swept through thousands of villages. Inundated by referrals, we reported [Reference Chopra14] a unique spectrum of rheumatic MSK (RMSK) following CHIKV illness and speculated a large emerging burden of MSK sequelae in the community.
Against this background, we completed a rural study to record the natural history of acute CHIKV and its RMSK sequelae. A population-based survey was followed by a planned longitudinal observational phase.
METHODS
Encouraged by patient referral pattern during the epidemic, we chose a village for the study.
Study site
Bavi village (1450 population, District Sholapur, state of Maharashtra) is situated about 200 km southeast of Pune and remains isolated and primitive. Farming is the predominant occupation and most people live in thatched-roof huts. Hygiene and sanitation conditions are primitive, and rainfall is scanty. Villagers carry water from deep wells over long distances. During the survey, we were often bitten by mosquitoes and one of our colleagues contracted acute Chikungunya. The village does not have any kind of medical facilities or a doctor.
Project team
The team comprised of three rheumatologists, three rheumatology assistant doctors, one microbiologist, one clinical research coordinator, three laboratory technicians, and four health workers. Several school-educated volunteers from Bavi assisted and were paid modest honoraria.
Patient selection and clinical evaluation
A house-to-house survey was conducted from 1 September 2006 to 14 November 2006. The screening question was ‘Have you suffered from any kind of fever since 1 March 2006?’ Some sporadic cases of suspected CHIKV were first reported from this region in March 2006. Respondents were interviewed as per the protocol questionnaire and asked to mark their pain sites on a human mannequin. Doctors examined all cases and recorded findings in a standard rheumatology-oriented case-record form. Acute onset of short duration fever with simultaneous polyarthralgias and body aches and pains was an a-priori definition used to initiate investigation of clinically suspected CHIKV cases. Malaria and dengue were excluded in all cases as these are known to be endemic in the region. We were further guided by the classical text descriptions of CHIKV [Reference Thiboutot3]. A definite diagnosis required serological evidence. MSK pains were further subclassified clinically into broad-based rheumatological classification categories of non-specific arthralgias (NSAs), inflammatory arthritis (IA), soft-tissue rheumatism, degenerative arthritis, and others. Standard classification criteria were referred [Reference Klipper15].
After obtaining verbal consent, blood samples were collected. Most cases in the village were treated symptomatically by neighbouring village doctors. It is difficult to perform data collection for long-term programmes in the community. As per protocol, we acquainted several local doctors regarding the aims of the survey and performed a basic health education programme about CHIKV in the village through small community group sessions. The education programme also ensured goodwill and cooperation from the villagers. When requested, we did treat several cases, free of cost, with analgesics (usually paracetamol) and NSAIDs (usually ibuprofen or diclofenac).
Follow-up
We did not find a new case of CHIKV in the village after completing the survey. Post-survey, all cases with persistent MSK pain of at least 4 weeks' duration were enrolled into an inception cohort and followed at 4- to 6-week intervals during the initial 9 months and at 8- to 12-week intervals thereafter. Patients were followed until resolution of symptoms or up to 18–24 months. A standard rheumatology case-record form was used. Blood samples were collected.
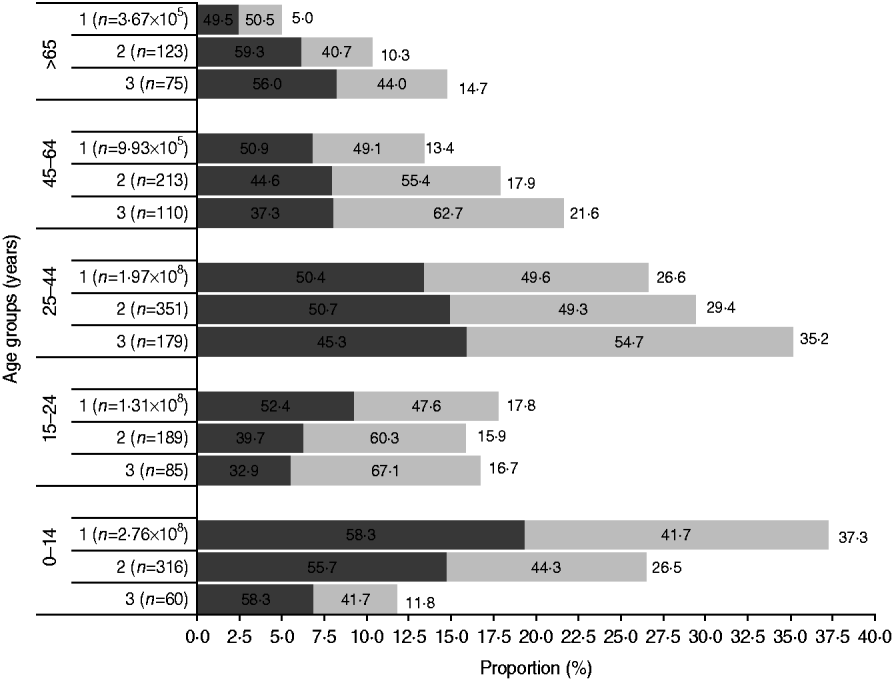
Fig. 1. The age and gender distribution in the Bavi village survey and rural Indian population (Census 2001) were comparable. Females (![]() ) outnumbered males (
) outnumbered males (![]() ) except in the children and elderly age groups. 1, Rural India; 2, Bavi survey population; 3, Bavi survey cases.
) except in the children and elderly age groups. 1, Rural India; 2, Bavi survey population; 3, Bavi survey cases.
Laboratory methods
Blood samples were transported to the Centre for Rheumatic Diseases: Research & Diagnostic Laboratory (CRD: R&D Lab), Pune, within 6–8 h under cool (not frozen) conditions. Serum was immediately stored at −80°C. Routine laboratory examinations were completed within 24 h. Blood samples from all cases were examined to exclude malaria (peripheral blood smears) and dengue (serology for IgM antibodies) and further for routine haematology and biochemistry, erythrocyte sedimentation rate [ESR, Westergren method, reported as fall (in mm) at the end of the first hour], C-reactive protein (CRP, using nephlometry; cut-off value 5 mg/l) rheumatoid factor (RF, estimated by nephlometry; cut-off value 40 IU/ml), anti-cyclic citrullinated peptide (anti-CCP, commercially available second-generation ELISA, Euroimmun, Germany; cut-off value ⩾5 RU/ml), antinuclear antibody (ANA, using qualitative enzyme immunoassay, anti-dengue antibodies (IgG and IgM, on-site lateral flow chromatographic immunoassay; CTK Biotech Inc. [16] and anti-CHIKV IgM antibodies (on-site CHIKV IgM rapid lateral flow chromatographic immunoassay, CTK Biotech Inc.). Anti-CHIKV IgG antibody test was performed using indirect immunofluorescence technique (Euroimmun Medizinische Labordiagnostika, Germany). The DuoSeT ELISA Development kit (R&D Systems, USA) was used to measure natural and recombinant human interleukin 6 (IL-6).
‘On Site Chikungunya IgM rapid assay’ was validated (sensitivity 95·6%, specificity 97%) by comparing the results of the first 137 blood samples to those of MAC ELISA performed at the National Institute of Virology, Pune.
Coincidentally, Bavi was close to our rural project site (Bhigwan) for an ongoing WHO ILAR (International League of Associations for Rheumatology) COPCORD (community-oriented programme for control of rheumatic diseases) since 1996 [Reference Chopra17]. Blood samples collected from consenting healthy villagers examined during COPCORD in 2005 (a year prior to the CHIKV epidemic of 2006) were used as healthy controls.
Database, analysis and statistical methods
Data entered into MS Excel sheets were analysed using SPSS version 12.0 (SPSS Inc., USA); with level of significance at P<0·05. The geometric mean was computed for sera cytokine data. Significant differences between groups were tested by ANOVA. In the post-survey period, we allowed a window period of ±2 weeks to adjust sample sizes shown in this report.
The study and the protocol were approved by the Centre for Rheumatic Diseases, Pune ethics committee.
RESULTS
In total, 1192 (response rate 82·2%) villagers were surveyed leading to identification of 509 cases (male:female ratio 0·8:1) of acute CHIKV illness. Overall, females outnumbered males and this difference was greatest in the 15–24 years age group (female:male 2:1). However, there were more male cases in children and the elderly (>65 years). The attack rate was 42·7%. Figure 1 shows the age sex distribution of population and cases and a comparison is made with the Indian rural population census 2001.
Survey cases (Fig. 2)
There were 110 (22%) cases examined within a fortnight of illness onset. Table 1 describes the features of acute illness. Arthralgias, predominantly peripheral, were disproportionately more severe than myalgias and soft-tissue pains (spine and proximal limbs). Fever generally lasted <5 days and was preceded by chills. Patients often complained of intense fatigue. The skin rash was faintly erythematous macular, mostly over arms and legs, and mildly pruritic and was difficult to identify in the dark-skinned complexion of the population. Knee (81·3%) was the most frequent pain site (Table 2). Pain/tenderness of the joint without swelling (overt synovitis) was the predominant feature. Hands, wrist and ankles often appeared mildly puffy probably due to tenosynovitis. There was generally a marked reduction in MSK pain by the end of the first week. Eighty-six percent of cases were classified as NSA, while 8·6% cases suffered from soft-tissue rheumatism that included regional (ankle tenosynovitis, foot fasciitis, heel pains) or a more diffuse scattered pattern; several were tender for fibromyalgia points (<8) but none fulfilled the criteria for classification of fibromyalgia [Reference Klipper15]. Twenty-nine (6%) cases were classified as inflammatory polyarthritis which was mostly confined to hands, wrists and feet. Among the latter, there were three known cases of rheumatoid arthritis (RA) with severe relapse (following CHIKV) and the remainder were best classified as undifferentiated in view of lack of diagnostic clinical criteria to diagnose a well defined rheumatic disorder (like RA or seronegative spondyloarthritis).
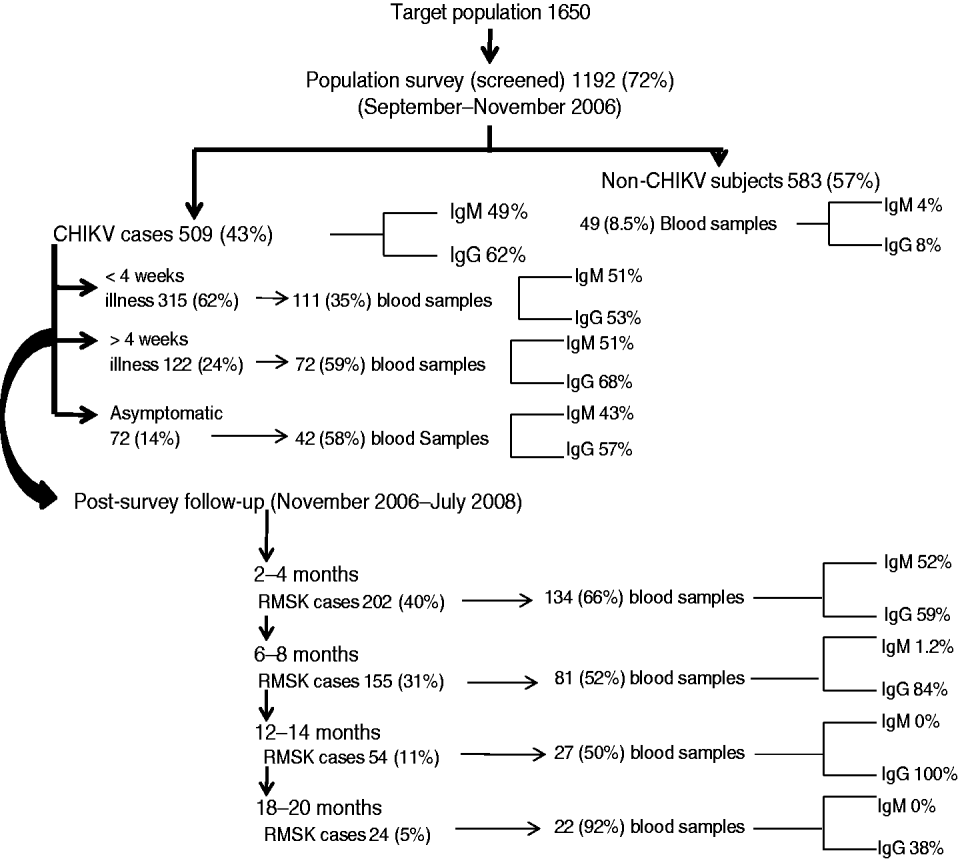
Fig. 2. Population survey and follow-up events to identify acute cases and those with persistent rheumatic musculoskeletal (RMSK) pain and disorder sequelae along with seropositivity for anti-CHIKV antibodies (IgM and IgG) in a prospective study of the acute Chikungunya epidemic in Bavi village (India).
Table 1. Clinical cases of CHIKV (n=509) in the Bavi survey: comparing selected features of acute illness (first week of illness) between children, adults and the elderly – distribution of number cases (frequency percent in parentheses)
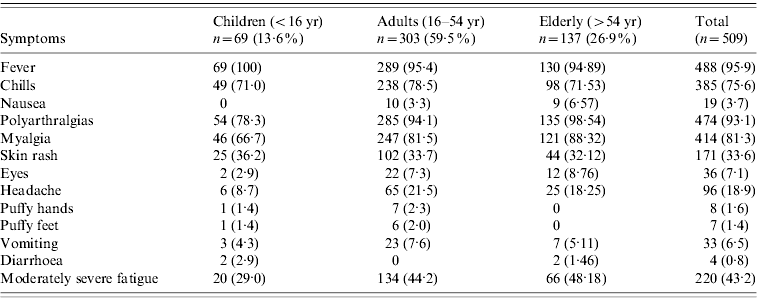
Table 2. Frequency (percent) of pain sites in patients suffering from persistent rheumatic musculoskeletal pains according to duration since CHIKV infection
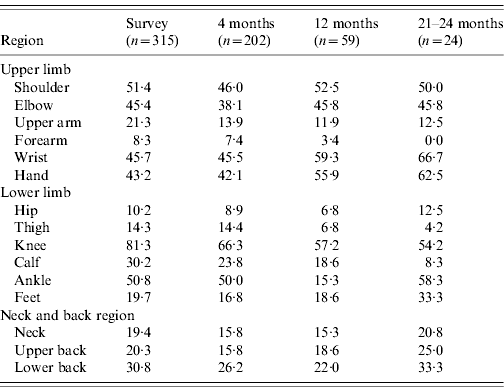
The overall profile of acute CHIKV (Table 1) did not differ in children, adults and the elderly. However, children with fever and MSK pains were often found playing in the field. None of the children ever showed IA. It is our clinical observation that overall children appeared to suffer less from the intensity of the acute illness, In contrast, elderly patients often looked very sick and remained confined to bed for 4–6 days. Of the survey cases, 19% and 42%, respectively, recovered within 14 and 30 days of onset of illness. We did not observe any fatality.
Long-term pain and chronic RMSK
There was complete recovery (as perceived by the patient) in 6% and 17% of survey cases within 1–2 months, and 2–4 months, respectively, while 16% continued to suffer beyond 4 months.
Table 2 shows the distribution of pain sites during the survey, and post-survey follow-up (4, 12, 24 months). Knee was the commonest pain site. Fifty-nine cases [12%, 95% confidence interval (CI) 8·9–14·7] and 24 cases (5%, 95% CI 3·0–6·9) identified during the survey suffered from MSK pains and arthritis at 1 and 2 years, respectively; the corresponding population prevalence was 4·1% (95% CI 3·11–5·22) and 1·6% (95% CI 1·1–2·5). The corresponding mean age of cases was 48 and 43 years; female:male ratio was 3:1 at both time points. MSK pains and arthralgias were predominantly non-specific. Although several non-specific cases also showed features of symptomatic osteoarthritis (OA) in weight-bearing joints and spine, the overall extent of arthralgias and soft-tissue aches and pains were sufficient to justify the classification of NSA; OA was a co-existent disorder which sometimes predated the onset of CHIKV. Excluding RA, the point prevalence of undifferentiated IA was 0·3% (95% CI 0·11–0·80) and 0·07% (95% CI 0–0·38) at 1 and 2 years, respectively.
Laboratory results
Figure 2 shows the frequency of seropositive anti-CHIKV IgM and IgG antibodies at different time points. Serum IgM antibodies could be detected on day 4 of clinical infection. Within 15 days of illness onset, 36% and 48% of cases, respectively, were seropositive for IgM and IgG antibodies. Seropositive IgM and IgG responses were found in 4% and 8% of villagers who did not recall a history of acute CHIKV. There were no significant differences in the clinical profile of acute illness and RMSK between patients who were seropositive and seronegative for anti-CHIKV IgM or IgG antibody (data not shown). We did not perform any other investigation for virus identification.
None of the survey cases tested positive for malaria parasites. Two of the five blood samples that tested seropositive for dengue antibody (IgM/IgG) were also seropositive for anti-CHIKV IgM antibody. None of the latter cases showed low platelet counts. Two (4%) healthy cases (control) tested seropositive for anti-dengue IgG antibody.
Thirteen cases tested seropositive only for RF (range 40–160 IU/ml); 11 NSAs, one known RA and one OA. One case of NSA was seropositive for both RF and ANA (40 IU/ml). Thirteen cases were seropositive only for ANA (10 NSAs, two undifferentiated IA, and one known RA). The frequency of seropositive RF and/or ANA did not increase during the follow-up assays and none of the naive seropositive cases (RF/ANA) developed IA during the study period.
None of the cases had anaemia, leucopenia or thrombocytopenia. It is prudent to add that routine haematological total and differential cell counts could not be performed during the first 3–4 days of illness and transient reductions in white cell count and platelets [Reference Sudeep and Parashar11] might have been missed. Serum assay for creatine kinase, renal and liver function tests were normal in all cases and remained so during the follow-up phase.
Although cases did not always fast, the mean value of ESR (Westergren) in the survey cases was 75 mm at the end of the first hour. Thirty-one (13·8%) cases were seropositive for CRP with a median value of 12 mg/l (range 6–96). The median serum assay of IL-6 in the survey cases was 262 pg/ml and this was 7·8 times higher than the corresponding value from the healthy control group (not statistically significant, P=0·097). Serum IL-6 remained elevated during the study period as shown in Figure 3 and was not associated with severity of pain or any particular rheumatic disorder.
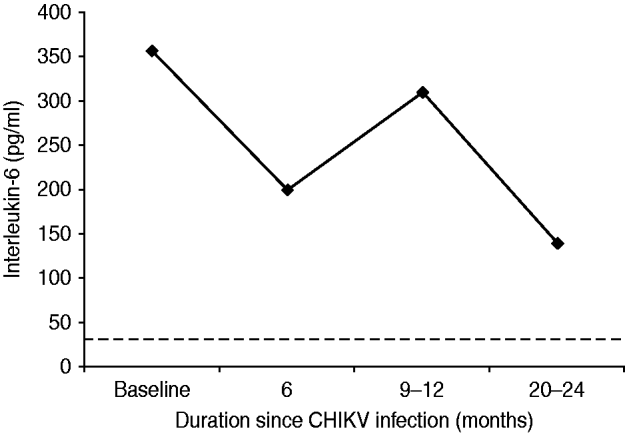
Fig. 3. Mean (geometric) of serum interleukin-6 assay in cases with persistent rheumatic musculoskeletal pains following acute Chikungunya illness in Bavi village. Baseline values coincide with clinical cases (n=225) identified during an epidemic survey in September–October 2006. (The horizontal dotted line represents mean values of healthy control subjects.)
DISCUSSION
Forty-three percent of the rural population in the current study suffered from symptomatic CHIKV illness during the epidemic. The clinical phenotype of a febrile onset of predominantly self-limiting severe MSK illness was consistent with earlier description [Reference Powers and Logue2, Reference Broom, Cook and Zumla4, Reference Pialoux5] and recent Indian reports [Reference Selvavinayagam18, Reference Kannan19]. Women outnumbered men in the cases and this was consistent with other Indian reports [Reference Selvavinayagam18, Reference Kannan19]. In the first month of illness, two-thirds of cases were seropositive for CHIKV antibodies (IgM/IgG) and two-thirds of cases recovered. Sixteen percent and 5% of cases suffered from chronic MSK pains and arthritis (mostly non-specific) beyond 16 weeks and 2 years, respectively. None died due to CHIKV in the current study. Some fatal cases have been reported from the epidemic in the French territory of Reunion Islands [Reference Sissoko20] but it is believed that comorbid diseases and drug toxicity rather than CHIKV per se predominantly contribute to mortality [Reference Pialoux5, Reference Sudeep and Parashar11].
This is possibly the first prospective clinical evaluation of persistent MSK and rheumatic disorders following CHIKV. The description is likely to be comprehensive as the study was performed by a rheumatology team. Severe chronic IA following CHIKV has been described from the earlier epidemics [Reference Brighton, Porzesky and De La Harpe7, Reference Kennedy, Fleming and Solomon21]. In an earlier study [Reference Chopra14] we reported an onset of several severe rheumatological disorders, ranging from RA to seronegative spondyloarthritis and psoriatic arthritis, following CHIKV and examination in a rheumatology referral centre. We have also reported the prevalence of undifferentiated IA (0·33%, 95% CI 0·49–1·1) and other forms of arthritis from this rural region of India [Reference Joshi and Chopra22]. Intriguingly, we did not find a single case of naive moderately severe IA in the Bavi rural community and only mild forms of undifferentiated IA were observed. The latter is surprising because a high proportion of CHIKV cases continued to suffer from moderately severe chronic MSK pain and arthralgias with elevation of acute-phase reactants (CRP, IL-6) for prolonged periods. The latter may be related to the genetics of the community.
In Bavi, anti-CHIKV IgM antibodies were demonstrated to persist for up to 6 months. In apparently healthy villagers without a history of CHIKV illness, 4–8% also tested positive (IgM or IgG) indicating that a certain load of infection in the community may be subclinical and asymptomatic. This vindicates our selection of healthy controls from the region in the year prior to the epidemic. We do not have data about the anti-CHIKV antibody status in this community prior to the epidemic but assays performed on 80 sera samples collected in 2005 from a neighbouring village, Bhigwan, did not show anti-CHIKV antibodies (A. Chopra & V. Anuradha, unpublished data). Both IgM and IgG CHIKV antibodies could be demonstrated within 6 days of onset of illness in the current study. This is rather unusual as it is uncommon to detect IgM antibodies within 4 days of illness [Reference Lahariya and Pradhan8] and IgG antibodies during the acute phase [Reference Kam23]. In another Indian report [Reference Lakshmi24], 48·6%, 55·4%, 21·5% and 62·5% of blood samples, respectively, tested positive for CHIKV using reverse transcriptase–polymerase chain reaction (RT–PCR), reverse transcriptase–light isothermal loop amplification (RT-LAMP), IgM (ELISA), and virus isolation in 296 clinically suspected cases of CHIKV. In a more recent report [Reference Lanciotti25] of Indians developing CHIKV illness on returning to USA after visiting India, 42·8% and 21·4% were found seropositive for IgM and IgG antibodies, respectively, within 10 days of the illness. RF can be false positive in several infections including viral [Reference Mackay, Leskovsek and Rose26] and its incidence in a normal healthy Indian population was reported to be <8% [Reference Anuradha and Chopra27]. We expected an increase in RF and ANA in the community following CHIKV. However, data of the current study demonstrated a marked low seropositivity for RF and ANA autoantibodies and anti-CCP. During follow-up (Fig. 2) all cases with persistent RMSK were repeatedly checked for RF and ANA but no increase in seropositive frequency was observed. This is consistent with our earlier study of referral patients with more severe phenotypes [Reference Chopra14]. IL-6 stimulates hepatocytes to produce CRP and both are proxy markers for inflammation. CRP and IL-6 are routinely found elevated in active RA and other IA [Reference Ballou, Kushner and Harris28]. Although both were elevated in the current study, IL-6 response (Fig. 3) was disproportionately higher and this discrepancy is difficult to explain. Perhaps this higher level is a unique host response to CHIKV and may be connected with persistence of virus in chronic cases. However, the peak levels seen at 9–12 months did not reflect any meaningful increase in the clinical severity of illness. We are unaware of any published study in the world that has demonstrated persistent elevation of IL-6 for over 18 months in patients with chronic post-CHIKV rheumatism. Although the clinical severity of arthralgias following CHIKV reduced over time, IL-6 remained several-fold higher than in healthy controls and probably demonstrates systemic inflammatory responses to CHIKV. The elevated serum IL-6 levels appear to last much longer than the symptomatic phase.
The attack rate (43%) of the virus in the current study was conspicuously high but within the range (4–45%) reported in Indian studies [Reference Lahariya and Pradhan8]. However, those studied were not based on real-time reporting from large sample size population surveys. Interestingly, a CHIKV outbreak [Reference Padbidri and Gnaneswar29] with an attack rate of 38% due to an ‘Asian variant’ was reported in 1973 from Barshi. Coincidently, Barshi is only 50 km from Bavi (the current study site). However, a new ‘African strain’ of CHIKV was identified as being responsible for the recent 2006 CHIKV epidemic in India [Reference Yergolkar30]. Therefore, the experience from Barshi and Bavi, widely separated in time, suggests that both the CHIKV strains were equally pathogenic in a naive population.
The pathogenesis of CHIKV [Reference Kam23, Reference Sourisseau31, Reference Couderc32] and the chronic arthritis and rheumatism sequelae remain unclear. Age (>45 years), severe initial joint pain and presence of underlying OA were reported to be the predictors of chronic MSK pain in a retrospective study of cases with chronic rheumatism following CHIKV [Reference Borgherini33]. In the current study, we did not perform an analysis for predictors. However, cases with persistent MSK pain in the current study were uniformly distributed across the 45–65 years age group and all recorded severe polyarthralgias at onset. Moreover, <10% cases were considered to be suffering from clinical OA during the survey. Although several arboviruses cause MSK pain, the clinical profile and outcome differs [Reference Vassilopoulous and Calabrese34]. Persistence of virus was demonstrated to explain the persistence of IA following Ross River arboviral illness [Reference Morrison35]. CHIKV RNA has been demonstrated in joints, muscle and skin during the acute phase of infection in animal models [Reference Sourisseau31], and human muscle satellite cells 3 months after infection [Reference Ozden36]. The current study is insufficient to provide a likely explanation for chronic MSK pains and rheumatic disorders following CHIKV. We speculate that persistence of virus rather than an autoimmune inflammatory mechanism probably explains the chronic MSK aches and pains in the study community. The extremely low frequency of autoantibodies (related to IA) and IA in the current study also support this contention.
The principal strength of the current study is its community base and support and a specialized rheumatology approach with sufficient blood sample collection. Importantly, the study commenced during the epidemic. A limitation of the study is that the logistics (time and distance travelled by the medical team) made it difficult for the medical team to capture acute events (especially onset of illness) and track down all cases; villagers would often leave early for the fields and return late. Several cases, especially when asymptomatic, refused laboratory investigations. We did not perform investigations to identify the virus, study vector and other environmental factors and genetics (human leucocyte antigen typing in particular).
A larger population study is required to determine the true extent of post-CHIKV rheumatism especially in respect of serious arthritis disorders reported previously [Reference Chopra14]. Moreover, a longer follow-up of the Bavi population may determine whether CHIKV per se leads to a higher incidence of arthritis.
ACKNOWLEDGEMENTS
This work was supported by Indian Council of Medical Research (Government of India) (5/8/7/20/2006-ECD-I) and Arthritis Research Care Foundation (CRD, Pune). Several colleagues in CRD, notably Dr Vaijayanti Lagoo Joshi, Dr Vinay Kunjeer, Dr Minaz Modak, Dr Harbeer Ahedi and Dr Sheetal Salvi assisted in examination of the patients. A dedicated CRD staff team (paramedics, nurses, laboratory technicians) endured hardship while travelling from Pune to Bavi and Modnimb. Several blood samples were initially tested for CHIKV IgM antibody at the National Institute of Virology, Pune. We thank the villagers of Bavi and Modnimb for their wholehearted support and generous cooperation, allowing completion of the project. We are grateful to Dr Lisa F. P. Ng (SIgN, Singapore) for reviewing some of the data and the manuscript. Finally, we acknowledge with gratitude the invaluable assistance and guidance of Professor C. S. Pandav (AIIMS, New Delhi) in preparing and reviewing the manuscript.
DECLARATION OF INTEREST
None.









