Crimean-Congo haemorrhagic fever (CCHF) is a severe disease caused by a tick-borne virus belonging to the Bunyaviridae family. It has a reported mortality rate of 3–30%. It has the most extensive geographic range of the medically significant tick-borne viruses, occurring in parts of Africa, Asia, Southeastern Europe, and the Middle East.
An epidemic in Iran was first confirmed in June 1999. Nosocomial transmission has been described in previous reports from Iran. In 1999 a physician acquired the disease from her husband, also a physician, who contracted the disease after contact with a hospitalized patient in the province of Chahar Mahal Bakhtiari and she died from CCHF. In 2001, another physician became infected after attending a father and son (both butchers) who had been hospitalized as suspected CCHF cases in the city of Isfahan; both were later confirmed as CCHF-positive. On this occasion the physician recovered [Reference Chinikar, Ergonul and Whitehouse1]. According to a survey carried out between June 2000 and September 2005 by the Laboratory of Arboviruses and Viral Haemorrhagic Fever of the Pasteur Institute of Iran, the most affected region in Iran was Sistan-va-Baluchestan in the southeast of the country. In Iran, the highest incidence was in August and September. However, in Khorasan only seven confirmed cases and two confirmed fatal cases were diagnosed. Mashhad is the second largest city in Iran and is located in the Khorasan Razavi Province, close to the borders of Afghanistan and Turkmenistan [Reference Chinikar, Ergonul and Whitehouse1]. The aim of this report is to describe a nosocomial outbreak of CCHF of six cases, of which two were fatal.
Table 1. Individual data for each confirmed patient
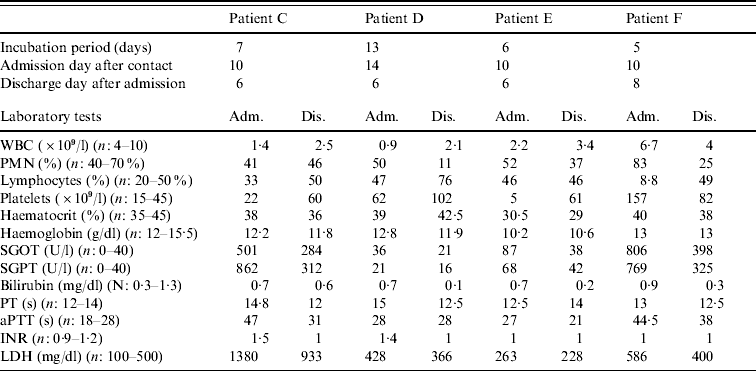
Adm., Admission; Dis., discharge; WBC, white blood cell count; PMN; polymorphonuclear cells; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; PT, prothrombin time; aPTT, activated partial thromboplastin time; INR, international normalized ratio; LDH, lactate dehydrogenase.
Patient A, a pregnant woman, presented at the Department of Gynaecology, Ghaem Hospital, Mashhad, Iran, with fever and epistaxis for one day. Following admission, she remained febrile, and developed shivering and bleeding from various sites of her body. Three days later, because of her worsening general condition, she was admitted to the intensive care unit but died of disseminated intravascular coagulation.
Patient B, a 31-year-old pregnant woman, had been referred from the countryside to the Ghaem Hospital because of elevated blood pressure. Unfortunately she was admitted to the same room and given the same bed that patient A had occupied before the room had been thoroughly disinfected. Three days later she developed fever, malaise and spotting. Laboratory investigations revealed leucopenia, thrombocytopenia and elevated liver enzymes. A provisional diagnosis of haemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome was made and the patient was treated accordingly. No further diagnostic testing was performed. Four days later, following a miscarriage, she developed severe vaginal bleeding. An emergency hysterectomy was performed, but the patient died of disseminated consumptive coagulopathy. An autopsy revealed considerable internal bleeding within various organs and tissues, including the liver, myocardium and lungs.
Patient C, a 50-year-old woman, was admitted to the Department of Infectious Diseases, Imam Reza Hospital, with fever, malaise and vaginal bleeding. She was a gynaecologist who had received a needle-stick injury while attempting to perform a hysterectomy on patient B 10 days previously. Seven days after the needle-stick injury she had experienced sudden fever, severe headache, nausea and vomiting. An infectious disease specialist who visited her at home had proposed hospitalization, but she refused. However, 3 days later, she was admitted to hospital because of persistent fever and vaginal bleeding. Laboratory tests performed prior to admission had shown leucopenia and thrombocytopenia (platelet count=22×109/l and WBC count=1·4×109/l). Physical examination revealed pale conjunctiva and relative bradycardia. She was treated immediately with ribavirin as CCHF was suspected, as well as imipenem and vancomycin for febrile neutropenia (the antibiotics were discontinued 3 days later). A transfusion of fresh frozen plasma (FFP) and platelets was given to correct her coagulation abnormalities. During her hospitalization, she developed hypotension and severe bradycardia (PR=40–50/min). A portable echocardiography was performed but this revealed no abnormalities. Three days after admission, she became afebrile, but nausea and vomiting persisted. Platelet and WBC counts gradually increased and she was discharged from hospital 6 days after admission with relative improvement of blood parameters and good general health.
Patient D was admitted 4 days after patient C. She was also a gynaecologist and presented to the Department of Infectious Diseases complaining of severe headache and muscle pain. She mentioned that she too had had percutaneous contact with patient B during the hysterectomy. She had begun self-treatment with a prophylactic dosage of ribavirin the day after developing a fever (the day before her admission). On admission, her liver functions [bilirubin=0·7 mg/dl, alanine aminotransferase (ALT)=21 U/l, and aspartate aminotransferase (AST)=36 U/l], were within normal range, but a complete blood count (CBC) revealed thrombocytopenia and leucopenia. CCHF was suspected and ribavirin was continued at a therapeutic dosage. During hospitalization her WBC count decreased to 1·2×109/l and platelet count to 5×109/l. She was finally discharged after 6 days as her symptoms had resolved. Laboratory data showed WBC count=2·1×109/l, platelet count=102×109/l, prothrombin time (PT)=12·5 s, and activated partial thromboplastin time (aPTT)=28 s.
On the same day that the patient D was admitted, two other patients were hospitalized in the Department of Infectious Diseases.
Patient E was a 26-year-old carer who had made contact about 10 days previously with patient B's contaminated clothes and sheets. She recalled a probable skin contact with the patient's blood while wearing a pair of perforated gloves. Four days prior to her admission she experienced a sudden onset of fever and myalgia. Headache and vomiting developed subsequently and she was referred to the hospital after an episode of epistaxis. Physical examination revealed some areas of ecchymosis on her trunk. Laboratory tests revealed prominent leucopenia and severe thrombocytopenia. Because of the similarities between the patient's history and the previous patients, ribavirin was prescribed. Serial investigations undertaken during the following days of hospitalization showed a gradual improvement in WBC count, platelet count and coagulation profile. The patient experienced no further bleeding, and was finally discharged after 6 days.
Patient F was admitted on the same day as patient E. She was also a carer and had also had several contacts with patient B, but stated that she had always used intact gloves when handling the patient or her body fluids. She had developed severe headache, nausea, intractable vomiting, and severe pain in her limbs and lower back for 5 days before admission. Laboratory investigations revealed a WBC count of 6·7×109/l, platelet count of 157×109/l, AST of 806 U/l, ALT of 769 U/l, aPTT of 44·5 s, and PT of 13 s. She was treated with ribavirin and supportive care, but her vomiting persisted for several days. She was finally discharged from hospital after 8 days.
A positive reverse transcription–polymerase chain reaction (RT–PCR) for the virus confirmed the diagnosis of CCHF in the last four patients, who completed a full 10-day course of ribavirin treatment without any complications. All four RT–PCR-positive samples were taken on the first day after admission of patients.
Nosocomial transmission of CCHF is well known, and has been described in outbreaks occurring in Pakistan, Dubai, and South Africa [Reference Harxhi2]. The most dangerous opportunities for acquiring the virus are interventions to control gastrointestinal bleeding, and emergency operations on patients not yet diagnosed. The risk of nosocomial spread is greater with severely ill patients who have a higher level of viraemia [Reference Mardani and Jahromi3]. The risks associated with various body fluids have not been well-defined, as most healthcare workers (HCWs) who have acquired infection have had several contacts with multiple fluids [4]. Mardani et al. studied the seroprevalence of anti-CCHF IgG in HCWs who had come into contact with CCHF patients from three referral hospitals in Systan-va-Baluchestan and Isfahan, the two provinces of Iran with the highest confirmed cases of CCHF in the 2001 outbreak; 3·87% of HCWs in the exposed group and none in the unexposed group tested positive for anti-CCHF IgG [Reference Mardani5]. Viral haemorrhagic fever (VHF) infection has not been reported in those whose contact with an infected patient occurred only during the incubation period [4]. Infectivity of the virus via usual routine contacts between patients and their family members and close relatives appears to be low in most reports [Reference Izadi6]. Harxhi et al., in an outbreak in Albania, concluded that the CCHF agent can be transmitted through apparently intact skin exposed to infected blood but, in the absence of skin defects or percutaneous injury with a contaminated device, exposure of mucous membranes through droplets or contaminated hands could play a more important role [Reference Harxhi2]. One of our patients (patient F) denied any cutaneous contact with patient A's or patient B's blood or other body fluids without barrier protection. However, she did not always use a face shield or surgical mask and eye protection. There is concern about airborne transmission of CCHF virus, although this mode of transmission has not been documented in humans [Reference Mardani5]. However, there are several reports of CCHF in those who had respiratory contact, but no direct contact with infected patients, animals or tissues [Reference Mardani5, Reference Sharifi Mood, Mardani and Metanat7]. Therefore, it remains unclear whether CCHF virus is transmissible via respiratory contact. CDC has recommended that if a patient with VHF has respiratory symptoms (e.g. cough or rhinitis), face shields or surgical masks and eye protection should be worn by HCWs to prevent droplet contact. However, epidemiological studies of VHF in humans indicate that infection is not readily transmitted from person to person by the airborne route [4].
All four patients who were HCWs and admitted to the Department of Infectious Diseases (patients C, D, E, F) were severely ill according to the severity criteria defined in Swanepoel [Reference Swanepoel and Gill8]. It is interesting that these four patients all had a notable bradycardia in relation to body temperature. They survived and were discharged from hospital with relative improvement of clinical manifestations and laboratory data.
Patient D received a prophylactic dose of ribavirin from the day she developed fever. Her clinical symptoms and laboratory abnormalities were significantly milder, supporting the efficacy of early administration of ribavirin in the course of the illness, even with a less than recommended therapeutic dosage. In a case-control study in patients with CCHF who had survived, ribavirin treatment had been instituted on average 24 h earlier, and about 2 days earlier in non-bleeding survivors than in bleeding survivors [Reference Izadi and Salehi9]. Late diagnosis decreases the efficacy of treatment and aggravates the outcome of the disease [Reference Ergonul, Ergonul and Whitehouse10].
It should be emphasized that the presumed diagnosis of CCHF for patient C during our nosocomial outbreak was made only on the basis of her percutaneous contact history with the blood of patient B, who had fever and bleeding. Subsequently, the involvement of three other patients who had had contact with body fluids of patient B suggested the occurence of a nosocomial outbreak of CCHF. Retrospective investigation of the course of illness, clinical signs and symptoms, laboratory test results and pathological findings at autopsy suggested a significant compatibility with the diagnosis of CCHF. At the time of writing this paper, autopsy specimens of patients A and B were not available for CCHF detection. Definitive testing to confirm or exclude CCHF in the first two patients (A and B) was not performed, mostly due to the physicians' unfamiliarity with the symptoms, signs and paraclinical findings of the disease in the Department of Gynaecology. In endemic areas, CCHF infection should be differentiated from HELLP syndrome. In CCHF infection, haemolysis is not seen and leucopenia is common, whereas in HELLP syndrome, haemolysis is seen and leucopenia is not common [Reference Crowcroft, Morgan and Brown11].
The pregnant patients in our study (patients A and B) developed severe manifestations of the disease and death occurred despite timely conservative treatment. In contrast, all four tertiary cases (patients C, D, E, F), who received ribavirin, survived. Our study may show that treatment with ribavirin in the course of CCHF is life-saving and even in pregnant patient outweighs the fetal risks (it has also been recommended for pregnant women with VHF of unknown cause [4]) (Table 1).
We therefore emphasize that, in endemic areas, each patient with a febrile haemorrhagic syndrome should be considered to have a viral haemorrhagic fever until proven otherwise. More important, other patients should not be placed in the same room occupied by a patient with fever and a suspected transmissible disease.
When bloodborne pathogens other than HBV or HIV are of concern, the Occupational Safety and Health Administration (OSHA) recommend the use of Environmental Protection Agency (EPA)-registered tuberculocidal disinfectants or hypochlorite solution. In our hospital sodium hypochlorite solutions are used to disinfect environmental surfaces routinely but unfortunately, this was not performed before patient B was admitted. Multiple studies in many countries have documented lack of compliance with established guidelines for disinfection and sterilization. Failure to comply with scientifically based guidelines has led to numerous outbreaks [Reference Rutala, Weber and William12].
Early diagnosis is not only important to prevent the spread of CCHF virus among HCWs and relatives of patients, but also makes early administration of ribavirin possible, thus reducing the clinical manifestations and improving the prognosis. For clinicians, an accurate risk assessment of a patient presenting with fever should be based on good medical intelligence. Medical intelligence includes all sources of information, such as surveillance and up-to-date reports on the situation in endemic areas and the precise mapping of epidemics [Reference Crowcroft, Morgan and Brown11].
ACKNOWLEDGEMENTS
We thank Ms. Veronika Aurens for editing and correction of the manuscript.
DECLARATION OF INTEREST
None.





