Growth is a marker of nutritional adequacy in pre-term infants when free of the major morbidities. Because postnatal growth failure is a common occurrence in these infants(Reference Embleton, Pang and Cooke1–Reference Fenton, McMillan and Sauve4) and is associated with increased morbidity and poorer neurodevelopmental outcomes(Reference Franz, Pohlandt and Bode5–Reference Latal-Hajnal, von Siebenthal and Kovari8), ensuring adequate growth through a nutritionally complete diet is vital. The effect of dietary long-chain PUFA (LCPUFA) on the growth of pre-term infants is controversial. Some early studies have suggested that supplementing pre-term formula with the n-3 fatty acids DHA (22 : 6n-3) and EPA (20 : 5n-3) reduced weight and length gains(Reference Carlson, Werkman and Tolley9–Reference Carlson, Cooke and Werkman11). An exploratory analysis(Reference Carlson, Werkman and Peeples12) from one of these studies(Reference Carlson, Cooke and Werkman11) showed a positive association between the concentration of erythrocyte arachidonic acid (AA, 20 : 4n-6) and weight and length, leading to the hypothesis that a ‘balanced’ dietary supply of n-6 and n-3 LCPUFA was necessary to support growth, and that AA should be added in infant formula when n-3 LCPUFA were present. However, subsequent studies of LCPUFA-supplemented formula that included AA and at higher concentrations than DHA have yielded inconsistent results. Studies have shown that LCPUFA supplementation had positive effects on weight(Reference Clandinin, Van Aerde and Merkel13–Reference O'Connor, Hall and Adamkin15) and length(Reference Clandinin, Van Aerde and Merkel13, Reference O'Connor, Hall and Adamkin15), no effect on weight(Reference Vanderhoof, Gross and Hegyi16–Reference Stier, Hess and Watzer18) or length(Reference Innis, Adamkin and Hall14, Reference Vanderhoof, Gross and Hegyi16–Reference Stier, Hess and Watzer18) or a negative effect on weight and length(Reference Fewtrell, Morley and Abbott19). Systematic reviews investigating the effects of LCPUFA-supplemented formula on the growth of pre-term infants have concluded that there are no clear effects of supplementation(Reference Simmer, Schulzke and Patole20, Reference Rosenfeld, Beyerlein and Hadders-Algra21). However, it is difficult to delineate the relative effects of DHA and AA as trials with DHA alone or DHA together with AA were combined. In addition, not all of the included studies were designed to assess growth, which requires multiple assessments over time.
The Docosahexaenoic acid for the Improvement of Neurodevelopmental Outcome in pre-term infants (DINO) trial was designed to evaluate the efficacy and safety of a dose of DHA that was estimated to match in utero accretion. The unique nature of the intervention allowed us to evaluate the effect of varying DHA supplementation when AA was held constant so that the ratio of DHA:AA ranged from about 1:2 in the control group to 2:1 in the higher DHA group. The present study reports the complete growth outcomes from the DINO trial. An earlier publication has demonstrated developmental benefits of higher DHA treatment with no negative clinical outcomes(Reference Makrides, Gibson and McPhee22).
This clinical trial has been registered with the Australian and New Zealand Clinical Trial Registry. The registration number is ACTRN12606000327583, and the trial web address is anzctr.org.au
Experimental methods
The methods have been reported previously(Reference Makrides, Gibson and McPhee22). Briefly, the DINO trial was a multi-centre randomised controlled trial conducted in five Australian perinatal centres. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the local human research ethics committees of each centre (Children, Youth and Women's Health Service, North Adelaide, SA, Australia; Flinders Medical Centre, Bedford Park, SA, Australia; King Edward Memorial Hospital, Subiaco, WA, Australia; Royal Women's Hospital, Parkville, VIC, Australia; Royal Brisbane and Women's Hospital, Herston, QLD, Australia). Written informed consent was obtained from all subjects.
Infants were excluded if they had major congenital or chromosomal abnormalities; were a multiple birth where not all live births were eligible; were in other trials of fatty acid supplementation or had a lactating mother where tuna oil was contraindicated (bleeding disorders, anticoagulants).
Interventions
Lactating women allocated to the higher DHA group took six 500 mg DHA-rich tuna oil capsules per d; if formula was required, infants were given a higher-DHA pre-term formula. Lactating women allocated to the standard DHA group took six 500 mg placebo soya oil capsules; if formula was required, a standard pre-term infant formula was used. This strategy resulted in infants randomised to higher DHA, receiving DHA triple that of infants randomised to standard DHA with AA remaining constant between the two groups. The actual concentrations of selected fatty acids in human milk and formula are reported in Table 1.
Table 1 Long-chain PUFA concentration in human milk and formula†‡
(Mean values and standard deviations of fatty acid as a percentage of total fat)
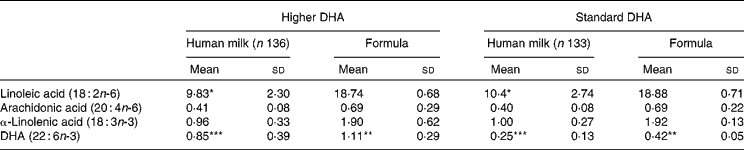
Mean values were significantly different: *P < 0·05, **P < 0·001, ***P < 0·0001.
† A 5 ml sample of breast milk was collected at the expected date of delivery appointment and immediately frozen at − 20°C.
‡ Infant formula and human milk fatty acids were analysed according to previously established methods(Reference Gibson, Neumann and Makrides28).
Outcomes
Weight, length and head circumference were measured at study entry, weekly until discharge home, at the expected date of delivery (EDD), and at 4, 12 and 18 months corrected age. The primary growth measures were absolute measures at 4, 12 and 18 months corrected age. Corrected age is calculated by subtracting the number of weeks born before 40 weeks of gestation from the chronological age. We also assessed rates of increase in weight, length and head circumference between study entry and EDD. Weight, length and head circumference were taken by trained research personnel. Weight was measured to the nearest 5 g on calibrated electronic scales. Length was measured using a recumbent length board with one person holding the infant's head in a vertical position, with the crown of the head touching the fixed headboard and a second person extending the legs and firmly placing the movable footboard against the infant's heels, and measured to the nearest 0·1 cm. Head circumference was taken as the largest occipitofrontal circumference, measured to the nearest 1 mm using a non-stretch tape measure. Data on milk or formula type consumed were collected during the intervention period and post-treatment at 4, 12 and 18 months corrected age. Timing of introduction of solids was collected at 4 months corrected age.
Randomisation
After receiving written informed consent, mother–infant pairs were randomised through a computer-driven telephone randomisation service. Stratification occurred by centre, birth weight ( < 1250 and ≥ 1250 g) and infant's sex. Multiple births were randomised according to the sex and birth weight of the first-born infant.
Blinding
There were four colour-coded groups: two for treatment and two for control. All capsules were similar in size, shape and colour. Formula was packaged by colour code. Parents, clinicians and all research personnel were blinded to the participant's study group.
Sample size
Sample size (n 657) was calculated to determine a clinically significant difference in the trial's primary outcome, the Mental Development Index of the Bayley Scales of Infant Development at 18 months corrected age(Reference Makrides, Gibson and McPhee22). This sample size allowed us to detect a weight difference of 395 g and a length difference of 0·86 cm with 80 % power. This represents 3·6 % of the weight and 1·1 % of the length at 18 months, respectively.
The study was planned to determine differences between sexes (because of the difference in the rates of growth between boys and girls) and in infants born weighing < 1250 g, as these are the most vulnerable in terms of growth. We had 80 % power to detect a 473 g difference in weight and a 1·3 cm difference in length in these subgroups.
Statistics
All infants were analysed according to the group to which they were assigned. The a priori level of significance was P < 0·05. Weight, length and head circumference measurements were converted to Z-scores (standard deviation scores) relative to the WHO Child Growth Standards(23) using WHO software(24). To account for dependence due to repeated measurements over time and multiple infants from the same mother, growth outcomes were analysed using linear mixed-effects models.
To assess the changes in absolute measures of weight, length and head circumference (both raw values and Z-scores) over the scheduled appointments, the effects of treatment group, time (EDD, 4, 12 and 18 months corrected age) and the interaction between treatment group and time were modelled. The differences in means at each time point (95 % CI) are presented, independent of the significance of the interaction effect, as these comparisons were a priori of interest.
To assess weekly growth in weight, length and head circumference, the effects of treatment group, time (corrected age at measurement) and the interaction between treatment group and time were modelled. Analyses of weekly growth were performed for the intervention period only (enrolment to EDD), as weekly data were available.
In the models, adjustment was made for the potential confounders of sex (raw scores) and gestational age at delivery (raw and Z-scores). A priori subgroup analyses based on infant's sex and birth-weight strata were planned as growth varies according to sex and birth weight. The subgroup analyses were performed via factorial linear mixed-effects models to allow testing for an interaction between treatment and subgroup. All calculations were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
The number of infants who were screened for the trial, randomly assigned to receive higher DHA or standard DHA and had growth assessments at EDD, and at 4, 12 and 18 months corrected age are shown in Fig. 1. Most trial management effort was directed at securing high follow-up rates for the primary neurodevelopmental outcome at 18 months corrected age (614 infants, 93·5 % of those enrolled)(Reference Makrides, Gibson and McPhee22). Growth was a secondary outcome, and where obtaining growth data proved more difficult, in particular at 12 months corrected age, this was left optional for families and is reflected in the lower rate compared with the high follow-up rates at EDD, and at 4 and 18 months corrected age. For example, weight data were available for 634 infants at EDD, this represents 97 % of the infants who were originally enrolled in the trial; at 4 months corrected age, 615 infants (94 %); at 12 months corrected age, 471 infants (72 %) and at 18 months corrected age, 598 infants (91 %). Despite this, the study represents the largest growth study of DHA supplementation in pre-term infants.

Fig. 1 Participant flow through the trial including number of infants assessed for each outcome (weight, length and head circumference) at each time point.
The trial began on 4 April 2001, and ended with the 18 months corrected age follow-up on 21 September 2007. The demographic and clinical characteristics of the infants and their families at randomisation were comparable between the two groups (Table 2). Median duration of treatment was comparable between the higher DHA and standard DHA groups (9·4 (interquartile range 7·9–11·4 weeks) v. 9·4 (interquartile range 8·0–11·6 weeks), respectively). Maternal adherence based on capsule returns was 81·1 % in the higher DHA group and 81·7 % in the standard DHA group (P = 0·88).
Table 2 Baseline demographic and clinical characteristics
(Mean values, standard deviations, medians, interquartile ranges, and percentages)

Absolute measures of weight, length and head circumference
There were no significant differences in weight, length or head circumference at EDD, 4 or 12 months corrected age or in Z-scores (Table 3). At 18 months corrected age, the length of the higher DHA group was 0·7 cm greater compared with that of the standard DHA group (95 % CI 0·1, 1·4 cm, P = 0·02, adjusted for sex and gestational age; Z = 0·28, 95 % CI 0·02, 0·54, P = 0·04, adjusted for gestational age; Table 3). There were no significant differences in weight or head circumference at this time (Table 3).
Table 3 Weight, length and head circumference at expected date of delivery (EDD), and at 4, 12 and 18 months corrected age with Z-scores*†‡
(Mean values and standard deviations, with difference of means as the treatment effect, n and 95 % confidence intervals)

* Reference data for calculating Z-scores according to the WHO Child Growth Standards(23).
† P values for treatment group-by-time interactions were as follows: weight (0·40 unadjusted, 0·44 adjusted), weight Z-score (0·54 unadjusted, 0·56 adjusted), length (0·11 unadjusted, 0·13 adjusted), length Z-score (0·11 unadjusted, 0·09 adjusted), head circumference (0·29 unadjusted, 0·27 adjusted) and head circumference Z-score (0·62 unadjusted, 0·63 adjusted).
‡ Raw scores adjusted for gestational age and sex; Z-scores adjusted for gestational age.
A priori subgroup analyses based on the randomisation strata showed an interaction effect between dietary treatment and birth weight ( < 1250 or ≥ 1250 g) for weight (P = 0·01) and length (P = 0·04). For Z-scores, a birth weight strata × diet interaction existed for length only (P = 0·03).
Infants born weighing ≥ 1250 g and randomised to higher DHA had a significant increase in weight at 12 and 18 months corrected age compared with infants randomised to standard DHA (adjusted difference in means 338 g, 95 % CI 89, 587, P = 0·01; 571 g, 95 % CI 254, 888, P = 0·0004, respectively). At 4, 12 and 18 months corrected age, infants in the same birth-weight strata (born weighing ≥ 1250 g) and randomised to higher DHA had significant increases in length (adjusted difference in means 0·6 cm, 95 % CI 0·0, 1·2, P = 0·04; 1·1 cm, 95 % CI 0·4, 1·9, P = 0·004; 1·7 cm, 95 % CI 0·9, 2·5, P = 0·0001, respectively; Z = 0·37, 95 % CI 0·09, 0·65, P = 0·01; 0·46, 95 % CI 0·14, 0·77, P = 0·004; 0·59, 95 % CI 0·26, 0·91, P = 0·0004, respectively) compared with infants randomised to standard DHA. For infants born weighing < 1250 g, the groups did not differ in weight or length. Z-scores by birth-weight strata are presented in Fig. 2.

Fig. 2 (a, d) Weight, (b, e) length and (c, f) head circumference Z-scores by birth-weight strata ( < 1250 g (a–c) and ≥ 1250 g (d–f)) at expected delivery date (EDD), and at 4, 12 and 18 months corrected age. Values are means, with standard deviations represented by vertical bars. A birth weight × diet interaction for Z-scores existed for length (P = 0·03). Birth-weight strata Z-scores were statistically significantly different: *P = 0·01, **P = 0·004, ***P = 0·0004. □, Higher DHA; ▧, standard DHA.
There were no interaction effects between dietary treatment and sex for weight, length or head circumference.
Rate of increase in weight, length and head circumference from enrolment to expected date of delivery (during the treatment period)
From enrolment to EDD, there were no differences between dietary treatment groups in the weekly growth rate in any of the growth indices such as weight, length and head circumference. There was a significant interaction effect (P = 0·01) between dietary treatment and birth-weight strata for the rate of head-circumference gain between enrolment and EDD. The head circumference of infants born weighing < 1250 g and randomised to higher DHA was 0·017 cm/week greater than that of infants randomised to standard DHA (95 % CI 0·003, 0·030, P = 0·02, adjusted for gestational age and sex).
There were no interaction effects between dietary treatment and birth-weight strata for the rate of increase in weight and length. Similarly, there were no interaction effects between dietary treatment and sex for the rate of increase in weight, length or head circumference.
Post-treatment diet (expected date of delivery to 18 months corrected age)
There were no differences between randomised groups in the post-treatment source of milk at 4, 12 or 18 months corrected age (Table 4). There was no significant difference in the percentage of infants who had been introduced to solids at 4 months corrected age (59·9 % higher DHA, 53 % control; adjusted relative risk 1·13, 95 % CI 0·97, 1·33; P = 0·12).
Table 4 Milk or formula type post-intervention
(Number of infants and percentages)

* Source of milk compared between groups using log-linear Poisson regression models.
† Adjusted for gestational age and sex.
Discussion
In this large-scale trial, we found that infants randomised to the higher-DHA diet were longer by 0·7 cm at 18 months corrected age compared with infants fed according to current best practice. This effect was modest (28 % of SD) and occurred 18 months after the intervention finished. Although it is tempting to speculate that this effect was due to random error, the direction is consistent with effects observed in some of the pre-planned subgroup analyses by randomisation strata. Infants born weighing ≥ 1250 g responded to the intervention differently from those infants with birth weight < 1250 g in the post-intervention period. There were no differences in post-intervention growth in infants born weighing < 1250 g, and those born weighing ≥ 1250 g and randomised to higher DHA had greater mean weights at 12 and 18 months corrected age and greater mean lengths and mean length Z-scores at 4, 12 and 18 months corrected age. It is unclear that why there was no effect on post-intervention growth in the lower birth-weight infants ( < 1250 g). These infants are sicker and grow less well generally.
However, during the treatment period from enrolment to EDD, infants born weighing < 1250 g and randomised to higher DHA had a significantly greater rate of head growth than those randomised to standard DHA. Such a small difference in the rate of head growth (0·017 cm/week, 95 % CI 0·003, 0·030; 0·2 cm over the course of average length of hospitalisation of 12 weeks) would seem unlikely to be of clinical significance. Nevertheless, it was in this group of infants where, in unadjusted analyses, a significant increase in the mental development index was found(Reference Makrides, Gibson and McPhee22). Observational studies in very pre-term infants have demonstrated an association between the rate of head circumference growth and improved neurodevelopment in childhood(Reference Franz, Pohlandt and Bode5, Reference Kan, Roberts and Anderson25, Reference Ehrenkranz, Dusick and Vohr26). It is possible that even such small, yet statistically significant, increases in the rate of head growth may be associated with neurodevelopmental improvement.
At the very least, our data indicate that higher DHA in the diet of pre-term infants has no negative effects on weight, length or head circumference during the first 18 months of life, despite the extreme change of LCPUFA balance in the early diet of pre-term infants. Unlike other studies, measurements of blood fatty acid status in our infants confirmed that the higher-DHA diets had resulted in a reduction in the plasma and erythrocyte membrane phospholipid AA concentrations as well as an increase in DHA and EPA in the higher DHA group compared with the standard DHA treatment(Reference Smithers, Gibson and McPhee27). Furthermore, although both groups of infants received dietary AA, the reduction in plasma and erythrocyte phospholipid AA observed in the higher DHA group implies that there was an exchange of AA for DHA and EPA incorporation into phospholipids. The results of the present large study with a robust design refute the reports of earlier studies, which suggested that this process may be a factor influencing poor growth in pre-term infants(Reference Carlson, Werkman and Tolley9–Reference Carlson, Werkman and Peeples12).
In comparison with WHO growth reference data that define ideal growth of children born at term, raised in a good environment and exclusively breast-fed, the weight and length of infants born weighing < 1250 g remained below the expected mean Z-score value of zero, although the growth of these infants improved with time. Conversely, the mean head circumference Z-score remained consistently above zero. In contrast, the growth of infants born weighing ≥ 1250 g was consistent with the WHO standard. While these data indicate that modern neonatal feeding practices result in post-term growth comparable with healthy, term infants for infants born weighing ≥ 1250 g, there remains room for improvement with regard to feeding practices for infants born weighing < 1250 g.
Many factors influence growth including the genetic potential of each infant, the in utero and ex utero environment, including diet, infections and medical treatments. The proportion of infants fed breast milk or formula, the type of formula and the proportion of infants fed solid foods did not differ between groups in the present study; however, more detailed information on dietary intake was not collected.
In this trial of DHA supplementation, we have shown that high dietary DHA intakes, capable of suppressing AA tissue incorporation, do not adversely affect the growth of pre-term infants. This observation may differ from other studies because all infants received some dietary AA or because some other studies had methodological limitations that could not exclude the possibility of bias or random error(Reference Carlson, Werkman and Tolley9–Reference Carlson, Werkman and Peeples12). The modest, positive effects of the higher-DHA diet on different measures of growth observed in the birth-weight strata are worthy of further investigation, while the consistent lack of effect by infant's sex indicated that higher-DHA diets have no differential effect on growth by sex.
Acknowledgements
We thank the families, and the medical, nursing and research staff who participated in each participating centre (the Women's and Children's Hospital; Flinders Medical Centre; the King Edward Memorial Hospital; the Royal Women's Hospital; the Royal Brisbane and Women's Hospital) and the staff of the Child Nutrition Research Centre and of the Data Management and Analysis Centre, University of Adelaide, North Adelaide, Adelaide. In the past, M. M., R. A. G. and K. S. have conducted clinical trials funded by the formula industry. They have no financial interest in the production and sales of infant formula or nutritional supplements. M. M. served on scientific advisory boards for Nestlé, Fonterra, and Nutricia; R. A. G. served on scientific advisory boards for Wyeth, Fonterra and Nestlé; K. S. served on a scientific advisory board for Wyeth. Associated honoraria for M. M. and R. A. G. were paid to their institutions to support conference travel and continuing education for postgraduate students and early career researchers. The honorarium for K. S. was paid to her directly. The present study was supported by a grant from the Australian National Health and Medical Research Council (ID 250322). Treatment and placebo capsules were donated by Clover Corporation, and infant formula was donated by Mead Johnson Nutritionals and Nutricia Australasia. Research fellowships were from the National Health and Medical Research Council of Australia (M. M., R. A. G., P. G. D. and P. B. C.). None of the funding bodies or companies had any role in the study design or conduct; data collection, management, analysis or interpretation; or preparation, review or approval of the manuscript. M. M., R. A. G., A. J. M., P. G. D., K. S., P. B. C. and S. M. designed the trial. M. M., A. J. M., C. T. C., L. W. D., K. S., P. B. C., S. M. and P. R. were involved in the data collection. C. T. C., M. M., R. A. G., A. J. M., T. R. S., P. R., L. W. D., K. S., S. M. and P. G. D. conducted the data analysis and interpretation. C. T. C., M. M. and R. A. G. were responsible for drafting the manuscript; all authors critically reviewed the manuscript. Statistical analyses were conducted by T. R. S. under the supervision of P. R.










