INTRODUCTION
Rabies caused by rabies virus (RABV) is one of the most important and widespread zoonotic diseases in the world. Although rabies is preventable, an estimated 55 000 people die of rabies each year worldwide, with the majority of deaths occurring in Asia and Africa [1]. RABV (genus Lyssavirus, family Rhabdoviridae) is a RNA virus with a single-stranded, negative-sense genome of ~12 kb in length consisting of five genes in the order 3′-N-P-M-G-L-5′, which encode the nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and RNA-dependent RNA polymerase (L), respectively [Reference Tordo and Kouknetzoff2, Reference Wunner3].
Rabies has been one of the most important infectious diseases in China since the 1950s [Reference Zhang4, Reference Zhang5]. Despite the fact that comprehensive preventive measures (including dog vaccination and post-exposure prophylaxis following human exposure) have been taken in the past five decades, rabies is still a significant public health problem in China [Reference Zhang4, Reference Hu6, Reference Tang7]. During the past 6 years, 2000–3390 human cases were reported each year. Previous investigations have shown that the domestic dog was the principal vector for rabies in China, and almost 95% of human cases were associated with dog transmission [Reference Zhang4, Reference Hu6–Reference Zhang8]. However, RABV has also been isolated or detected in other animal species such as deer, wolf, cat, fox, pig, cattle, and ferret badger in China [Reference Zhang4, Reference Hu6, Reference Zhang9–Reference Hou13].
Genetic characterization of RABVs isolated from animals and humans indicates that there are two genetically distinct clades of RABV circulating in China [Reference Zhang10]. The viruses of the first clade (clade I), which are closely related to those from the Southeast Asian countries including Indonesia, Malaysia, the Philippines, Thailand, and Vietnam, are widely distributed in the endemic areas. However, the viruses of the second clade (clade II), which are closely related to the cosmopolitan viruses, are found infrequently. Recently, Arctic-like RABVs were found to be present in raccoon dogs in the far-eastern region of Russia and the Korean peninsula (Fig. 1) [Reference Hyun14–Reference Park, Shin and Kwon17]. Although raccoon dogs are widely distributed in the northeastern part and in the rabies endemic areas of China [Reference Zhang4, Reference Zhang18], no Arctic-like RABV has been identified in this species yet.

Fig. 1. The site of the rabies outbreak in Inner Mongolia, China (•) and geographic distribution of Arctic-like virus identified in the far-eastern region of Russia and the Korean peninsula (▴).
Here we describe the epidemiology of an outbreak of rabies in domestic raccoon dogs on one of the animal farms in Inner Mongolia in 2007. Genetic analyses of the strains isolated from rabid raccoon dogs suggest that the rabies outbreak was caused by Artic-like rabies virus.
MATERIAL AND METHODS
Rabies cases in raccoon dogs and sample collection
In 2007, rabies cases in raccoon dogs were defined by clinical signs and confirmed by detecting RABV antigen in animal brains obtained from the animal farm in Zhengxiangbaiqi County, the Inner Mongolia Autonomous Region (province), China (Fig. 1), which raised about 400 raccoon dogs (Nyctereutes procyonoides) and 200 silver foxes (a variant of red fox) (Vulpes vulpes) for fur production.
Brain samples were collected from rabid raccoon dogs and sent to the Institute of Special Wild Economic Animal and Plant Science of the Chinese Academy of Agricultural Sciences for detection of RABV antigen. Information such as date of onset of illness, clinical symptoms and signs, number of raccoon dogs and foxes, and history of the animal farm was obtained and recorded.
RABV antigen detection and virus isolation
RABV antigen in raccoon dog brain smears was detected by using direct immunofluorescence assay with the fluorescein-labelled anti-RABV N monoclonal antibodies (Fujirebio Diagnostics, USA) as described previously [Reference Zhang19]. For RABV isolation, a 20% brain suspension was made by homogenizing brain tissue in PBS supplemented with 0·75% BSA, penicillin (500 U/ml) and streptomycin (2 mg/ml). One-day-old suckling mice were inoculated via the intracerebral route and observed for 30 days for development of rabies. Brains were removed from dead mice that succumbed to rabies.
Reverse transcription–polymerase chain reaction (RT–PCR), DNA cloning and sequencing
Total RNA was extracted from raccoon dog brains and virus-infected mouse brains as well as from uninfected mouse brains for use as negative controls with TRIzol reagent according to the manufacturer's instructions (Invitrogen, China). In order to amplify the entire G gene sequences, primer RV-GFP (5′-ATGGTGCCATTAAACCGCTGCAT-3′) was used for cDNA synthesis at 94°C for 5 min and reverse-transcribed at 42°C for 60 min and 75°C for 10 min. PCR for amplification of the entire G gene was performed with primers RV-GFP and RV-GRP (5′-GTGAKCTATTGCTTATGTCCYTC-3′). For the entire N gene sequences, primer RV-NFP (5′-ATGTACCACCTCTACAATGGATG-3′) was used for cDNA synthesis, primers RV-NFP and RV-NRP (5′-CCYTGGAGATGAGCCTGAT-3′) were used for amplification.
The final PCR products were purified from gel slices by using an Agarose Gel DNA Purification kit (TaKaRa Biotechnology Co. Ltd, China) according to the manufacturer's instructions and subjected to sequencing. DNA sequencing was performed with the ABI-PRISM Dye Termination Sequencing kit (USA) and an ABI 373-A genetic analyser.
Phylogenetic analysis
Alignments were prepared with ClustalW version 1.83 (www.ebi.ac.uk/Tools/clustalw2/index.html). The nucleotide and amino-acid identities were calculated using the DNAStar program (DNAStar, USA). The PHYLIP program package (version 3.68) (http://evolution.genetics.washington.edu/phylip.html) was used to construct phylogenetic trees using the neighbour-joining (NJ) method with the Kimura two-parameter substitution model and the maximum-likelihood (ML) methods with Hidden Markov model (Ts/Tv ratio 2·00, constant rate for site variation) with 1000 bootstrap replicates. RABV gene sequences, including representative viral sequences from China and from around the world (obtained from GenBank, www.ncbi.nlm.nih.gov/Genbank), were used for comparison. The GenBank accession numbers of the sequences used in this study are shown in Figure 2.

Fig. 2. Phylogenetic tree based on open reading frame (ORF) nucleotide sequences of (a) N gene and (b) G gene of the Chinese rabies viruses and those from other countries with the neighbour-joining method using the PHYLIP program package (3.68). Bootstrap values for 1000 replicates above 50% are shown at the branch nodes. Sequences obtained from the current study are shown in bold.
RESULTS
Rabies cases at the raccoon dog farm
One wild raccoon dog was captured in the grassland not far from an animal farm in Zhengxiangbaiqi County in the middle of March 2007, and sent to the animal farm where it was raised together with other domestic raccoon dogs. Two weeks later, the wild raccoon dog became agitated and aggressive, and escaped from the animal farm, suggesting that this animal might be suffering from rabies.
Subsequently, an outbreak of rabies occurred in domestic raccoon dogs on the animal farm. The first raccoon dog, which was housed next to the wild raccoon dog, developed symptoms of rabies by the end of May. From May to October 2007, a total of 15 domestic raccoon dogs died of rabies. All these animals exhibited typical signs of central nervous system (CNS) disturbances. Sudden anorexia, signs of apprehension or nervousness, irritability, hyperexcitability (including priapism), and aggression were observed in all rabid raccoon dogs. However, no foxes, which were raised in cages separated from the raccoon dogs by a corridor, exhibited any signs of CNS disturbances or other clinical diseases. By October all remaining animals were vaccinated with rabies vaccine and no further rabies cases were found thereafter.
The animal farm is located in a field surrounded by a 2-m high wall. All raccoon dogs were housed in individual cages that were raised to a height of 1 m from the floor. There were 30 cages that were connected to one another in a single row. Contact and even bites between neighbouring raccoon dogs occurred frequently. All 15 rabid raccoon dogs were reported to have a history of contact with other rabid raccoon dogs. There was no report of rabies previously on this farm and no human or domestic animal rabies cases were reported on other neighbouring animal farms during this period.
Virus antigen detection and virus isolation
Brains were obtained from 5/15 rabies-symptomatic raccoon dogs. Viral N antigen was detected in all five samples (Fig. 3), which were all positive for rabies viral RNA by RT–PCR. Five virus isolates (designated NeiMeng925, NeiMeng927A, NeiMeng927B, NeiMeng1025B, NeiMeng1025C) were obtained by mouse inoculation. Virus isolation was further confirmed by RT–PCR.
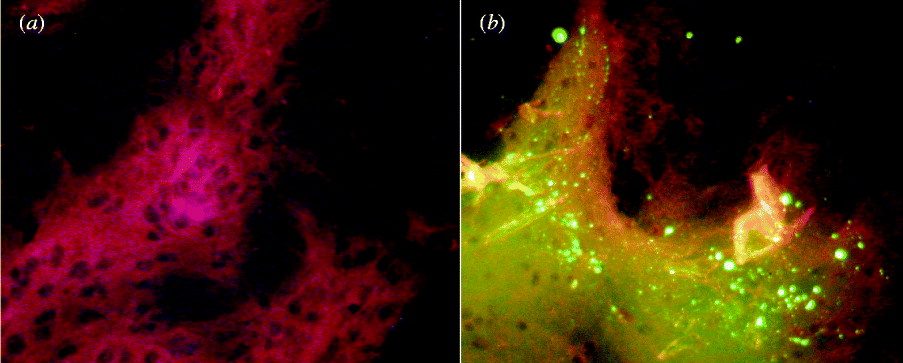
Fig. 3. Detection of rabies virus nucleoprotein antigens by direct immunofluorescent assay with anti-rabies virus N monoclonal antibodies. (a) Rabies virus antigen-negative mouse brain tissue. (b) Rabies virus antigen-positive rabid raccoon dog brain tissue. Magnification ×400.
Genetic analysis
To characterize these virus isolates, both the entire viral G gene sequences (GenBank accession numbers: EU284095, EU284096, EU284097, EU284098, FJ415313) and N gene sequences (GenBank accession numbers: EU284093, EU284094, EU652444, EU652445, FJ415313) were determined for all five isolates and subjected to genetic analysis. These sequences were very closely related to each other, with 99·9% nucleotide identity for the G gene sequences and 99·9–100% nucleotide identity for the N gene sequences. All the entire G gene and N gene sequences recovered from rabid raccoon dogs showed higher identity to those of Arctic and Arctic-like viruses (90·2–96·5% for the G gene sequences, 92–96·8% for the N gene sequences) and those of other RABVs associated with terrestrial animals around the world (86·5–87·9% for the G gene sequences, 88·4–89·9% for the N gene sequences). However, these viruses showed relatively lower identity to those in clade I, which are widely distributed in the endemic areas in China [Reference Zhang10, Reference Zhang19, Reference Meng20], with only 79·5–84·8% nucleotide identity for the G gene sequences and 83·1–88% nucleotide identity for the N gene sequences.
Phylogenetic analysis
In order to investigate the molecular linkage between the isolates from rabid raccoon dogs in Inner Mongolia with other known RABVs from China and around the world, we compared the open reading frame (ORF) nucleotide sequences of the N gene of our isolates with those of RABVs available in GenBank using the NJ method with the PHYLIP program package. As shown in Figure 2a, all isolates from the rabid raccoon dogs are closely related to viruses in genotype 1 (classic RABV), particularly to Arctic and Arctic-like viruses, well separated from other RABVs supported by a high bootstrap value (100%), indicating that these isolates belong to the Arctic-like RABV. Further analysis indicates that these isolates are highly related to Arctic-like-2 viruses isolated in raccoon dogs or foxes from the far-eastern region of Russia [Reference Kuzmin15, Reference Kuzmin16] and those isolated from raccoon dogs in South Korea [Reference Hyun14]. These isolates were relatively distinct from Arctic and Arctic-like-1 viruses [Reference Kuzmin16], and highly divergent from those in clade I, which are widely distributed in the endemic areas in China [Reference Zhang10, Reference Zhang19, Reference Meng20].
Overall, the phylogenetic tree based on the ORF nucleotide sequences of the G gene showed the clustering pattern similar to that based on the entire N gene sequences (Fig. 2b). Finally, the N- and G-based trees constructed using the ML method had a congruent topology to those constructed by the NJ method (data not shown).
DISCUSSION
In this study, we described the rabies outbreak in domestic raccoon dogs on an animal farm in Inner Mongolia, China in 2007. The rabid animals had typical clinical signs of rabies. Epidemiological investigation and genetic analysis of recovered virus genomes showed that the outbreak was caused by an Arctic-like RABV. This is the first report of Arctic-like RABV in China.
The province of Inner Mongolia is located southeast of the Mongolia plateau. It is a frontier area of north China, and shares borders with Mongolia and the far-eastern region of Russia (Fig. 1). Although Inner Mongolia is not one of the seriously affected areas in China [Reference Zhang4, Reference Zhang5, Reference Tang7, Reference Zhang10], 22 human rabies cases have been reported from 1998 to 2008. At present, no rabies has been identified either in domestic or wild animals because of a lack of active surveillance [Reference Wu21]. In this outbreak, a total of 15 rabies cases occurred in domestic raccoon dogs. This outbreak was probably transmitted by local wild raccoon dogs since no rabies cases had been reported on this or neighboring animal farms previously. The possible index case was the wild raccoon dog that was captured and placed together with other domestic raccoon dogs on the farm. Two weeks after the capture, the wild raccoon dog developed agitation and aggressiveness. Unfortunately, this wild animal escaped from the farm and rabies could not be confirmed; however, the symptoms observed in this animal suggest that it might have had rabies and thus transmitted the virus to other domestic raccoon dogs on this farm. This reasoning also suggests that RABV is present in the local wild raccoon dog population. Thus, it is recommended that captive wild animals including raccoon dogs and foxes should be quarantined and vaccinated with rabies vaccine. These wild animals should not be raised together with domestic animals. Since raccoon dogs are widely distributed in the northeastern part of China [Reference Zhang18], they may be another important source of infection for domestic animals or humans [Reference Zhang4, Reference Hu6–Reference Zhang8].
Previous studies indicated that Arctic RABVs are widely dispersed throughout the Northern Hemisphere [Reference Mansfield22–Reference Smith24]. Recently, molecular epidemiological studies have shown that Arctic and Arctic-like viruses are present in the Middle East, and southern and eastern Asia [Reference Hyun14–Reference Park, Shin and Kwon17, Reference Nadin-Davis25, Reference Nadin-Davis26]. In the present study, phylogenetic analysis of both the G sequences and the N gene sequences of five isolates obtained from rabid raccoon dogs indicated that these Chinese viruses were closely related to the Arctic-like viruses [Reference Hyun14–Reference Kuzmin16, Reference Nadin-Davis25], but divergent from those which are widely distributed in the endemic areas in China [Reference Zhang10, Reference Zhang19, Reference Meng20]. Our data indicate that the Chinese viruses isolated from raccoon dogs belong to the Arctic-like virus group, and the rabies outbreak was caused by Arctic-like virus. As these Chinese isolates are more closely related to Arctic-like-2 viruses isolated from raccoon dogs or foxes in the far-eastern region of Russia [Reference Kuzmin15, Reference Kuzmin16] and those isolated from raccoon dogs in South Korea [Reference Hyun14] than to Arctic-like-1 or Arctic viruses [Reference Kuzmin16, Reference Nadin-Davis25], our results also support the notion that RABVs from terrestrial mammals are phylogeographically dependent [Reference Bourhy27, Reference Davis28].
In conclusion, our study indicates that the rabies outbreak was caused by Arctic-like virus which might be from local wild raccoon dogs in Inner Mongolia. As Arctic-like RABVs are present in raccoon dogs and red foxes, which are widely distributed in China, this study reinforces the need for regular surveillance of local wild animals and vaccination of domestic animals in order to prevent rabies. Finally, captive wild animals should not be allowed contact with domestic animals.
ACKNOWLEDGEMENTS
We sincerely thank Dr Chang-Chun Tu and Dr Yu Jiang at Institute of Military Veterinary for assisting in the detection of rabies antigen and Dr Zhen F. Fu at Department of Pathology, College of Veterinary Medicine, University of Georgia for help in revision of the manuscript. We thank Dr Charles E. Rupprecht at Rabies Program, Division of Viral and Rickettsial Diseases, Centers for Disease Control and Prevention for his helpful advice on the manuscript. The study was supported by Public Welfare Research Fund (2005DIB4J048) from the Ministry of Science and Technology of China.
DECLARATION OF INTEREST
None.







