Introduction
Areas of human conflict may be important for wildlife because the exclusion of normal economic activities in such areas can result de facto in conservation (Martin & Szuter, Reference Martin and Szuter1999) and create effective protected areas (Higuchi et al., Reference Higuchi, Ozaki, Fijita, Minton, Ueta, Soma and Mita1996). However, the generality of this argument has been challenged and recent evidence, especially from forest-based conflicts in Africa, suggests a negative impact from over-harvesting of wildlife, degradation of habitats and pollution, and the prevention of a range of necessary conservation and protection activities (Dudley et al., Reference Dudley, Ginsberg, Plumptre, Hart and Campos2002; McNeely, Reference McNeely2003). Understanding the effects of conflict on wildlife has important implications for the way conservation agencies work in conflict areas (Plumptre et al., Reference Plumptre, Hart, Vedder and Robinson2000; Hanson et al., Reference Hanson, Brooks, da Fonseca, Hoffmann, Lamoreux and Machlis2009), especially how effectively they can respond at the cessation of hostilities (Draulans & Van Krunkelsven, Reference Draulans and Van Krunkelsven2002; McNeely, Reference McNeely2003) because the period of time immediately after war is often crucial (Dudley et al., Reference Dudley, Ginsberg, Plumptre, Hart and Campos2002).
Areas of high biodiversity appear to be particularly vulnerable to conflict (Hanson et al., Reference Hanson, Brooks, da Fonseca, Hoffmann, Lamoreux and Machlis2009). The Upper Guinea forests of West Africa (between Guinea and Togo) are in a region where three of the six forested countries have recently suffered armed conflict and unrest. These forests are of high global biological importance because they form one of the two main blocks of African tropical forest, with high levels of biodiversity and endemism and numerous threatened species (Stattersfield et al., Reference Stattersfield, Crosby, Long and Wege1998; Poorter et al., Reference Poorter, Bongers, Kouame and Hawthorne2004). These forests are considered a critical priority for conservation because only c. 5,000 km2 of pristine forest remains of a former total area of c. 50,000 km2 (Mittermeier et al., Reference Mittermeier, Robles Gil, Hoffmann, Pilgrim, Brooks and Goettsch Mittermeier2004). They are threatened by logging for timber, clearance for agriculture and mining, and hunting for the bushmeat trade (Brashares et al., Reference Brashares, Arcese, Sam, Coppolillo, Sinclair and Balmford2004). The impacts of war may exacerbate any or all of these pressures; however, there have been few studies that have documented such impacts.
Gola Forest in Sierra Leone, at the west of the Upper Guinea block, provides an opportunity to assess the impacts of war. It is the last remaining extensive tract of lowland forest in Sierra Leone and one of the most important remaining forests in the region (Davies, Reference Davies1987; Collar & Stuart, Reference Collar and Stuart1988; Allport et al., Reference Allport, Ausden, Hayman, Robertson and Wood1989; Fishpool & Evans, Reference Fishpool and Evans2001; Okoni-Williams et al., Reference Okoni-Williams, Thompson, Koroma and Wood2005) holding most of the region’s endemic, threatened and near-threatened mammals and birds (Davies, Reference Davies1987; Klop et al., Reference Klop, Lindsell and Siaka2008). Forest featured significantly in the 1991–2001 civil war in Sierra Leone (Richards, Reference Richards2005) and the Revolutionary United Front (RUF) established a number of camps in forested areas, including near Gola Forest. The RUF’s reliance on forest is made clear in their manifesto (Sankoh, Reference Sankoh1995), leading Squires (Reference Squires2001) to conclude that there must have been a significant negative impact on forest biodiversity. Gola Forest was the subject of a number of biological surveys prior to the war (Merz, Reference Merz1986; Davies, Reference Davies1987; Allport et al., Reference Allport, Ausden, Hayman, Robertson and Wood1989; Davies et al., Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007) and thus resurveys to assess changes during the intervening period may be valuable. We undertook biodiversity surveys in Gola Forest in 2006–2007 to determine the effects of the war on the forest (Klop et al., Reference Klop, Lindsell and Siaka2008) and report here how the results from the mammal surveys compare with pre-war data, providing the first assessment of change in a West African forest during a period of war.
Study area
Gola Forest in Sierra Leone lies along the border with Liberia between 7°18′ and 7°51′ N and 10°37′ and 11°21′ W. It is dominated by lowland moist evergreen high forest, with an annual rainfall of c. 3,000 mm mostly falling in a single wet season from May to October. The woody vegetation is dominated by Leguminosae–Caesalpinoideae, Euphorbiaceae, Leguminosae–Mimosoideae and Sterculiaceae (Klop et al., Reference Klop, Lindsell and Siaka2008). Three forest reserves were gazetted from the 1930s onwards, consisting of four forest blocks (Fig. 1). Gola West (c. 67 km2) and Gola East (c. 205 km2) are low-lying and swampy (mean altitudes of 131 and 152 m, respectively). Gola North (c. 417 km2) and its Extension 2 (c. 61 km2) are more rugged and higher than the surrounding landscape (mean altitude 303 m). The forest is drained by the Moa River to the west and the Mano and Moru rivers to the east. Tiwai Island is a low-lying sandy island (mean altitude 120 m) of c. 12 km2 in the Moa River, close to Gola West. It has been the subject of intensive biological research in the past (Oates et al., Reference Oates, Whitesides, Davies, Waterman, Green, Dasilva and Mole1990) and we consider it briefly here.
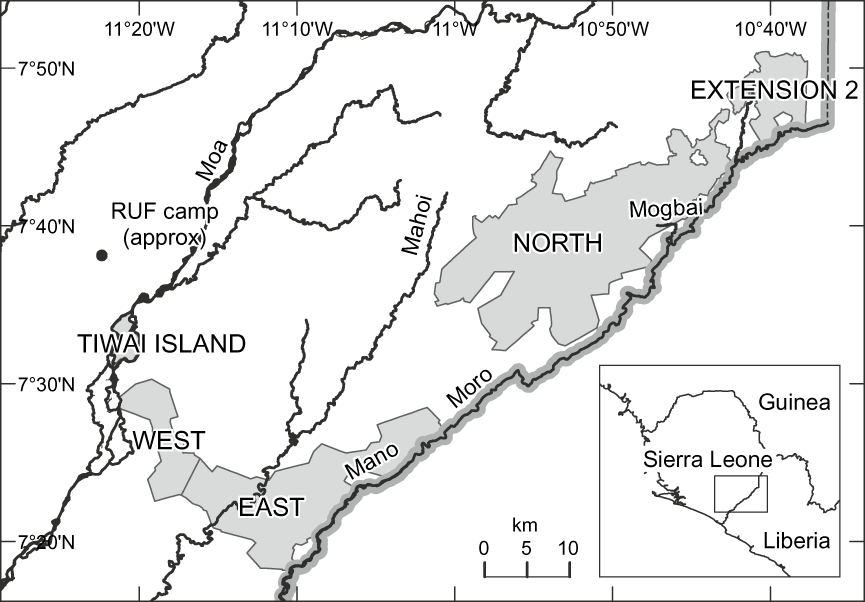
Fig. 1 The Gola Forest reserves (shaded) and Tiwai Island, with main rivers and the national border with Liberia. Inset shows the location of the main map in West Africa.
Methods
Our post-war surveys were based on line transect distance sampling (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). Lines transects are considered the most efficient way to sample wildlife in African forests (Plumptre, Reference Plumptre2000) although for certain groups such as nocturnal duikers other methods may be preferable (Davies et al., Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007). Transects were randomly superimposed onto the study area in a systematic segmented grid sampling design (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2004), stratified by forest block area to account for variation in habitat quality among the blocks. Transect fragments at the reserves’ boundaries were discounted for logistical reasons. Forty-eight transects were surveyed, 28 twice, totalling 245.3 km of survey effort: 23.8 km in Gola West, 72.0 km in Gola East, 129.3 km in Gola North and 20.2 km in Extension 2. All transects were surveyed on foot by two observers between December 2005 and April 2007. All encounters were recorded, including signs (e.g. dung, footprints, nests), and the position along the transect was noted to the nearest 25 m. Distances to sightings were measured using a laser rangefinder (accuracy ± 1 m) and bearings were recorded with a compass.
Encounter rates per km were calculated from visual and aural records. Primate group densities were calculated from group sightings only, using Distance v. 5.0 (Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010). Perpendicular distances were left ungrouped and the upper 5% of distances truncated. Primate observations were pooled for analysis and post-stratified by species. Population densities were estimated by multiplying group densities by mean group size. Estimates of group size based on single sightings of unhabituated primate groups may be inaccurate (Thomas, Reference Thomas1991) and line transect counts can underestimate group size (Defler & Pintor, Reference Defler and Pintor1985; Thomas, Reference Thomas1991). Mean group sizes from Tiwai Island (Oates et al., Reference Oates, Whitesides, Davies, Waterman, Green, Dasilva and Mole1990) were therefore also used. These were based on intensively studied groups, and previous estimates of primate abundance on Tiwai Island (Fimbel, Reference Fimbel1994) and in Gola Forest (Davies et al., Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007) used the same method. Primate biomass was calculated from published mean group weights (Oates et al., Reference Oates, Whitesides, Davies, Waterman, Green, Dasilva and Mole1990). Additional information was obtained from ad hoc observations, bushmeat markets, informal interviews with local hunters and from two other short post-war surveys in Gola Forest (Anderson et al., Reference Anderson, Shorrock and Hunter2007; Dowsett-Lemaire & Dowsett, Reference Dowsett-Lemaire and Dowsett2007).
Pre-war data came from systematic surveys of elephant Loxodonta cyclotis dung along transects (Merz, Reference Merz1986), transect-based surveys of all mammals and signs (Merz, Reference Merz1986; Davies, Reference Davies1987), mapping of calling primates in plots (Davies, Reference Davies1987; Davies et al., Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007) and sweep samples of primates in 50-ha plots (Davies et al., Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007). For the latter, group densities were converted to population densities using mean group size data from Tiwai Island (Oates et al., Reference Oates, Whitesides, Davies, Waterman, Green, Dasilva and Mole1990). Whitesides et al. (Reference Whitesides, Oates, Green and Kluberdanz1988) found good agreement between this method and distance sampling on Tiwai Island.
We compared density estimates, encounter rates, discovery rates, occupancy levels of the forest blocks and species lists between pre- and post-war surveys to assess change that occurred because of the civil war. None of the pre-war surveys reported confidence intervals and therefore statistical comparisons were not possible. The analysis considered only the larger mammal species, being those most commonly taken as bushmeat, and so excluded small insectivores, bats, and all rodents other than squirrels (Sciuridae), anomalures or scaly-tailed squirrels (Anomaluridae) and porcupines (Hystricidae). The taxonomy and names of the mammals follow Duff & Lawson (Reference Duff and Lawson2004) and threat status follows IUCN (2010).
Results
Post-war surveys recorded 44 species of larger mammal, including 18 threatened, Near Threatened and endemic species (Table 1). Only two threatened species thought by Davies (Reference Davies1987) to occur in Gola Forest (golden cat Felis aurata and Jentink’s duiker Cephalophus jentinki) and four common species that he recorded with certainty were not relocated. Jentink’s duiker has since been found (Ganas & Lindsell, Reference Ganas and Lindsell2010). Seventeen species were found that Davies (Reference Davies1987) did not record, four of which were new records for Gola Forest (Grubb et al., Reference Grubb, Jones, Davies, Edberg, Starin and Hill1998).
Table 1 Larger mammals recorded in the Gola Forest reserves, with their IUCN Red List (2010) status, whether endemic and the origin of the record.

1 EN, Endangered; VU, Vulnerable; NT, Near Threatened; LC, Least Concern; DD, Data Deficient
2 New records for Gola Forest
The discovery rate of new species on the transects (Fig. 2) was the same as that reported by Davies (Reference Davies1987), with 26 species in the first 40 days. The proportion of the three forest reserves occupied by each species increased significantly from pre- to post-war (Wilcoxon = 442.033, P = 0.004).
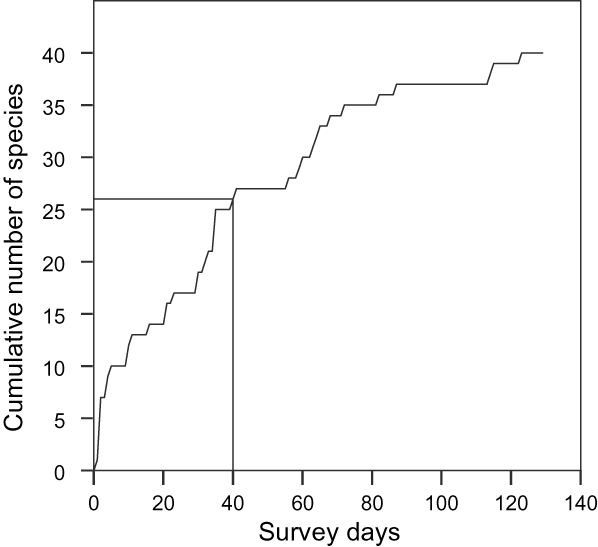
Fig. 2 Species accumulation curve for post-war surveys in the Gola Forest reserves (Fig. 1). The reference lines show the total number of mammals recorded and survey effort during a pre-war survey (Davies, Reference Davies1987).
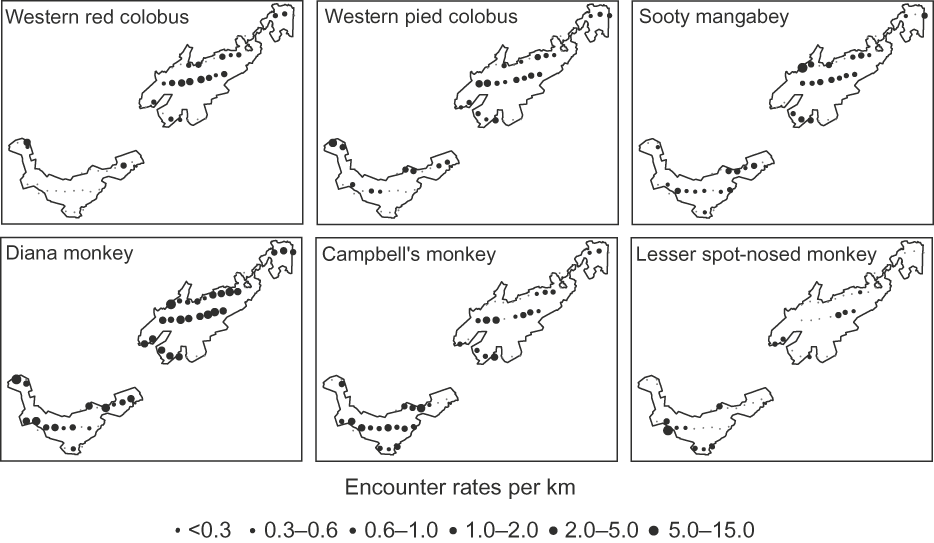
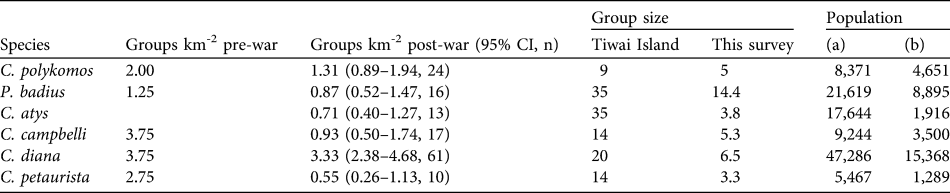
Ten primate species were recorded post-war, including one ape and two prosimians. Numbers of the Endangered western red colobus Piliocolobus badius were high but the species was not ubiquitous (44% of transects), preferring the less disturbed areas of Gola North (Fig. 3) where the encounter rate was > 10 times higher than in the more heavily logged and hunted Gola West and Gola East (Kruskal–Wallis X 3 = 16.0, P = 0.001). The group density estimate was 0.87 km-2 (Table 2), which gives a population estimate of 8,895 using our mean group size or 21,619 using the mean group size from Tiwai Island.
Table 2 For six species of monkey (Cercopithecidae, Table 1) in Gola Forest, the density of groups per km2 pre- and post-war (latter with 95% confidence interval, CI, and n), group size estimated for Tiwai Island in the 1980s and obtained during this survey, and population estimates based on (a) mean group sizes for Tiwai Island, which are probably overestimates for Gola and may have changed in the intervening years, and (b) mean group sizes from this survey, which are probably underestimates given the difficulty of estimating group sizes adequately during line transect surveys.
Endangered chimpanzees Pan troglodytes were only detected by vocalizations (n = 25) and thus population estimates were not possible and comparison with pre-war surveys difficult. However, calling animals were detected in Gola East, Gola North and Extension 2 at a relatively consistent rate (0.107, 0.117 and 0.138 km-1, respectively) suggesting widespread occupancy, as was found by Davies (Reference Davies1987).
The Vulnerable Diana monkey Cercopithecus diana was the most abundant and widespread of all monkeys in the forest, with 187 records from 83% of the transects and a mean group density of 3.33 km-2. Sooty mangabey Cercocebus atys and western pied colobus Colobus polykomos, both Vulnerable, were also abundant and widespread (Table 2) but the only Near Threatened primate, olive colobus Procolobus verus, was the most seldom encountered monkey (four records) and perhaps poorly surveyed by these methods. Two widespread species, which were more common at forest edges, Campbell’s monkey Cercopithecus campbelli and lesser spot-nosed monkey Cercopithecus petaurista, were found at relatively low densities within the reserves (Table 2).
Of the four threatened species of monkey, group densities differed little from pre-war estimates for Diana monkey and western red colobus (in Gola North only) but were lower for western pied colobus (Fig. 4). A comparison could not be made for sooty mangabey because its density was not estimated by Davies et al. (Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007). Campbell’s monkey and lesser spot-nosed monkey, which are both common and widespread species in Sierra Leone, had much lower densities post-war (Fig. 4).

Fig. 4 Comparison of pre- and post-war group densities of six species of monkey (Cercopithecidae, Tables 1–2) in Gola Forest (Fig. 1). Post-war circles include 95% confidence interval error bars. WRC, western red colobus; WPC, western pied colobus; SM, sooty mangabey; DM, Diana monkey; CM, Campbell’s monkey; LSM, lesser spot-nosed monkey.
Pooling the primate data and using group sizes from Tiwai Island indicated an overall primate biomass of 638 kg km-2. Gola North had the highest level, at 830 kg km-2, which was similar to that found by Davies et al. (Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007) on their plot in the Mogbai area of Gola North (875 kg km-2).
Distance data were insufficient for population estimates of any ungulates. Footprints and dung of forest elephants were found at five locations around the Mogbai River in Gola North but not elsewhere. This equates to an encounter rate of 0.04 km-1 in Gola North compared to 1.49 (± 0.93) from a 1982 survey in the same area (Merz, Reference Merz1986). There were two elephant populations in the mid 1980s, with an estimated 50 animals in Gola North and an estimated 60 in Gola East centred around the Mahoi River (Merz, Reference Merz1986). Signs were also found by Davies (Reference Davies1987) in Gola North and in the west and the east of Gola East.
Numerous signs of the Endangered pygmy hippopotamus Hexaprotodon liberiensis were found along two small rivers to the south of Gola North post-war (Anderson et al., Reference Anderson, Shorrock and Hunter2007), and animals were seen in this area on two occasions. Footprints and dung were also found on Tiwai Island, where the species is well-known, and reported from three other sites close to the reserves. Davies (Reference Davies1987) reported the presence of the pygmy hippopotamus around the Mahoi and Wemago rivers in Gola East and he thought it probably also occurred in the Mogbai area in Gola North.
Only Maxwell’s duiker Cephalophus maxwelli was recorded frequently, with 14 sightings during 244 km of surveys over 82 days. Pre-war, Davies et al. (Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007) reported sighting Maxwell’s duiker c. 70 times during 130 km of surveys over 51 days, 10 times the post-war rate. Using sweep samples he estimated a density of 30 km-2 for all duiker species in two forest plots. Numerous post-war records of duiker signs (n = 80) indicated that this group was much more abundant and widespread throughout the forest than sightings suggested.
Five other duiker species were recorded post-war, with a few records each confirming their continued presence in the forest and no marked change compared to pre-war records. In comparison, during 40 days of surveys, Davies (Reference Davies1987) recorded zebra duiker Cephalophus zebra twice, yellow-backed duiker Cephalophus silvicultor twice (on Tiwai Island) and bay duiker Cephalophus dorsalis in three localities but not black duiker Cephalophus niger or Ogilby’s duiker Cephalophus ogilbyi. The Upper Guinea form of Ogilby’s duiker, Brooke’s duiker Cephalophis ogilbyi brookei, was seen for the first time in Gola Forest on two occasions during our surveys, both times in the undisturbed forest of Gola North. Water chevrotain Hyemoschus aquaticus and African buffalo Syncerus caffer nanus were also recorded for the first time within the reserves and were widespread.
Discussion
Despite 10 years of civil war in Sierra Leone our results show that the mammal fauna of Gola Forest has survived relatively intact and that such forests can continue to be important sites for the conservation of threatened Upper Guinea forest wildlife after conflict. No large mammal species was extirpated during the war and previously unrecorded species have been discovered. Some of the most threatened species continue to have healthy populations in the forest, especially primates, and showed little or no sign of reduced abundance.
The most important loss we found was a large decline in elephant numbers. No evidence of elephants was found in Gola East or Gola West and only a few signs in Gola North. The former Gola East population is now presumed extinct, a conclusion confirmed by local hunters who had not seen elephants in Gola East since the war. The elephants in Gola North may not be permanently resident in this area but move across the Moro River to Liberia. Reports from villagers along the international border suggested that the number of elephants crossing the Moro River has decreased significantly, however, and that only a few individuals may now be present in Gola North. Merz (Reference Merz1986) argued that hunting and timber extraction were the most important factors threatening the elephants of Gola Forest and estimated an annual net population decline of 5%, which could account for the current low numbers. The crash in the Gola population brings the elimination of the forest elephant from Sierra Leone closer; it is known from only two other localities in the country, Tonkoli and Kangari. These populations were small even in the 1980s and under heavy pressure from hunting (Grubb et al., Reference Grubb, Jones, Davies, Edberg, Starin and Hill1998).
The areas used by pygmy hippopotamus to the south of Gola North are a mix of floodplains dominated by herbaceous vegetation and patches of riverine forest in a fairly remote area. There is very little information on the ecology of this species so it is hard to make inferences about likely population sizes, but it seems probable that several 10s of animals persist in and around the reserves. Although the home range of this species is only thought to be c. 1.5 km2 for males (Roth et al., Reference Roth, Hoppe-Dominik, Muhlenberg, Steinhauer-Burkart and Fischer2004), and thus numbers could be high, 63% of the riverine forest in the Gola area lies outside the reserves and the species may not therefore be well protected.
Primate group densities appear to be high but it is possible that mean group sizes in Gola Forest declined during the war so that current population size estimates based on pre-war group sizes from Tiwai Island (Oates et al., Reference Oates, Whitesides, Davies, Waterman, Green, Dasilva and Mole1990) would be inflated. Mean group sizes for arboreal monkeys measured during the resurvey were 24–56% of those published for Tiwai Island. For sooty mangabey, mean group size was just 11% of the value from Tiwai Island, reflecting the difficulty in counting group members of a terrestrial forest species. Furthermore, the group sizes reported from Tiwai Island do not necessarily represent the distribution of group sizes encountered during a systematic survey because they discount singletons, and it is unclear how animals unattached to established social units were accounted for. Improved mean group size estimates for use in systematic surveys are therefore still needed.
The sharp declines of lesser spot-nosed and Campbell’s monkeys are hard to explain. Although they were the most frequently hunted monkeys before the war (Davies et al., Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007) this was because most hunting took place in community forests and not in the reserves. Hunters who entered the forest were targeting the larger colobines (Davies & Richards, Reference Davies and Richards1992). It is possible that they have changed their behaviour and become more elusive. There is no evidence that the forest structure has changed and become less suitable for them.
Line transects were not effective for assessing the status of duikers and other small antelopes in the forest (Newing, Reference Newing1994; Davies et al., Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007). All expected species were still present but it is not possible to say whether numbers have increased or decreased. Duikers are a popular source of bushmeat and it is possible that they have been disproportionately selected; the low encounter rates for Maxwell’s duiker suggest so. They are more readily snared than primates and so can be hunted without the need for a firearm (possession of which is now illegal in Sierra Leone), and a significant sector of society in Sierra Leone does not eat primate meat. Maxwell’s duiker was the single most important ungulate in bushmeat sales and hunting bags in the Gola area (Davies et al., Reference Davies, Schulte-Herbrüggen, Kümpel, Mendelson, Davies and Brown2007), outweighing the most important monkey, in biomass terms, by 3–18 times. The paucity of felid records in our surveys may indicate a limited prey base. Firmer conclusions regarding the status of duikers will require additional data, based on different survey methods.
Gola Forest survived the war because of a set of circumstances peculiar to its geography. Although the RUF maintained bases in the forest reserves it seems no camps were actually in Gola Forest. Much of the RUF’s aggression was directed at the rural community, resulting in rural depopulation, and many people abandoned settlements around Gola Forest. Only a few people moved into the forest reserves to escape the rebels. The cross border trade in bushmeat with Liberia, which was prominent before the war (Teleki & Baldwin, Reference Teleki and Baldwin1981; C.M. Hill, pers. comm.), almost ceased as the international border closed and road travel became unsafe. Local markets also shrank (AMS, pers. obs.). Whilst Gola Forest may not have suffered serious negative impacts from the war, and may even have benefited from depopulation of people, it is likely that the impacts were displaced to other sites, such as Kambui North Forest Reserve adjacent to the town of Kenema. This forest acted as an emergency resource for the refugee population that fled to Kenema for protection; its wildlife was depleted and its timber cleared (AMS, pers. obs.). Forest reserves elsewhere in the country are likely to have suffered similarly (Squires, Reference Squires2001).
Although conservation activities are easier to undertake in the absence of hostilities, substantial threats to the forest emerged after the end of the war from logging and mining and from an increasing human population, all of which increase fragmentation of habitat and demands for bushmeat in the absence of alternative protein sources. This contrasts with elsewhere on the continent where recent levels of lawlessness, displacement of human populations and explicit targeting of threatened species during periods of conflict have posed a serious threat (Koenig, Reference Koenig2008). Whether other Upper Guinea forests in conflict areas have similarly survived is unclear at present. However, the example of Gola Forest demonstrates that such sites can retain important mammal populations despite the passage of war, and conservationists need to recognize the high priority of re-establishing conservation programmes as soon after such conflicts as is feasible.
Acknowledgements
We are grateful to the following colleagues in the Gola Forest Programme: Denis Bannah, Ibrahim Kanneh, Michael Kanneh, Alhassan Kemokai, Chief Joseph Kenneh, Patrick Lamboi, the late Morrison Lukulay, the late Mr Soso Samai, Prince Soriba, Ibrahim Swaray, Mohamed Swaray, Mohamed Sulay, John DeMarco, David Zeller, John Moriba, Gilbert Koker, James Allieu and Sheku Koroma. The Conservation Society of Sierra Leone director D.D. Siaffa, Chris Squire and Bartholomew Kamara provided support throughout. This work was funded by the UK Darwin Initiative, the European Union, Fonds Français pour l’Environnement Mondial, the Royal Society for the Protection of Birds, and Conservation International. We are grateful to Paul Donald, Debbie Pain, Alistair Gammell and two anonymous referees for comments.
Biographical sketches
Jeremy Lindsell focuses on tropical forest conservation in West Africa and Asia, managing surveys and carrying out threatened species research, monitoring and ecosystem services research. Erik Klop is an ecologist at Royal Haskoning in the Netherlands. He has undertaken field research on mammals and birds in West Africa, focusing on antelopes in north Cameroon and on the conservation of forest birds in Sierra Leone. Alhaji Siaka is the head of the Research and Monitoring department of the Gola Forest Programme and has a particular interest in forest bird conservation. He currently leads bird and mammal surveys and monitoring in Gola Forest.