Campylobacter is the leading cause of bacterial intestinal infection in the developed and developing world [Reference Blaser1], and infections appear predominantly sporadic. The most common exceptions are household outbreaks, which comprise 3–5% of infections as reported in Denmark [Reference Ethelberg2] and in Wales [Reference Ribeiro and Frost3].
Two-thirds of family-associated outbreaks in a region of South Wales (1996–1999) [Reference Ribeiro and Frost3] each contained a single strain, as defined by serotyping and phage-typing. But the identification of Campylobacter outbreaks is hindered by the co-occurrence of different Campylobacter strains in individual outbreaks [Reference Forbes4] and the lack of agreed portable typing methodologies [Reference Ethelberg2].
Multi-locus sequence typing (MLST) is a genotypic strain-typing method based on DNA sequences from seven housekeeping genes [Reference Dingle5] that has been applied to many bacterial species. The sequence types (STs) generated can then be used to compare strains and provide molecular evidence for identifying outbreaks. The aims of this study were to (i) identify putative household outbreaks in cases reported to national surveillance in Scotland, (ii) characterize their further epidemiological attributes, and (iii) evaluate genotypic strain composition as a means of confirming these outbreaks.
During July 2005 to September 2006, 5831 cases of human campylobacteriosis were reported to national surveillance in Scotland. Campylobacter isolates, from reported human cases, were submitted from public health bacteriology laboratories and typed by MLST as previously described [Reference Sheppard6]. Linkage between epidemiological data (address, age, sex and date of report) and MLST type was achieved for 3713 cases. Putative household outbreaks were defined as ⩾2 cases with the same residential address and family surname, and reporting dates within 28 days. Ninety-one such outbreaks were identified (86 with 2 cases, three with 3 cases and two with 4 cases), and these comprised 3·2% of all reported Campylobacter cases. According to a randomization test [Reference Manly7], these groups were significantly more frequent (P<0·00001) than the 6·4 groups expected to occur by chance in the 2·29 million households in Scotland during the study period [8]. During this period >30-fold household outbreaks were reported than general outbreaks (n=3). This is of the same order of magnitude to that found in Denmark where >21-fold household outbreaks were reported than general outbreaks [Reference Ethelberg2]. Further, it is worth noting that in Scotland the three general outbreaks comprised a total of 40 cases which suggests that there are about five times as many cases associated with household outbreaks. However, a larger study is required to confirm the robustness of this difference.
Twenty-six of these putative household outbreaks had complete MLST genotyping information (Table 1). In 23 (89%) of these putative household outbreaks the cases within each group shared the same ST, and this was significantly more often (P<0·00001) than expected by chance (13%). The high frequency of strain matching within the putative household outbreaks resembled the strain composition of family-associated outbreaks in Wales [Reference Ribeiro and Frost3]. From these results it can be hypothesized that the other five putative household outbreaks which contained different STs were caused by co-infection with different strains from the same source, e.g. food vehicle, as is known to have occurred in an outbreak attributed to chicken liver pâté [Reference Forbes4].
Table 1. Epidemiological data for putative household outbreaks genotyped by MLST sequence type
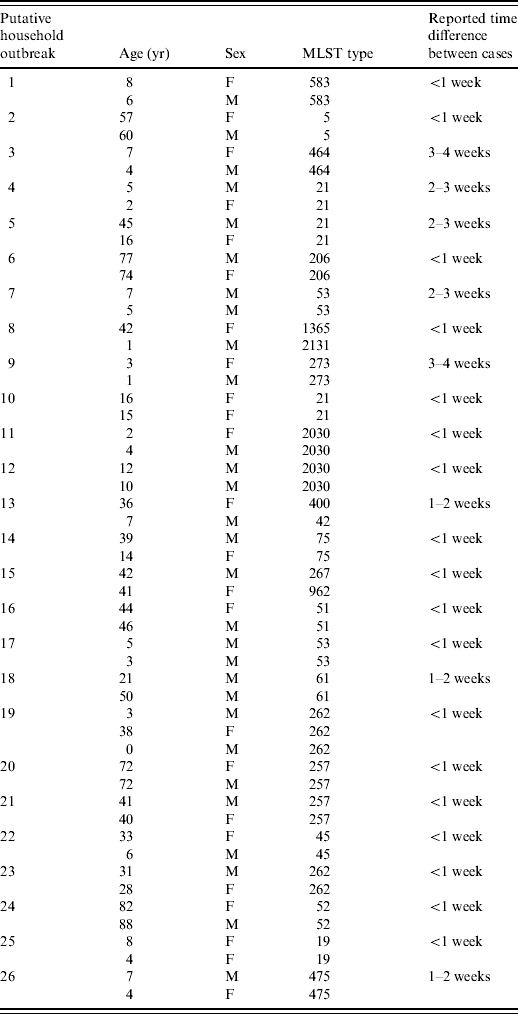
The cases in each putative household outbreak showed the following differences in reporting dates: 62% were up to 1 week apart, 20% were 1–2 weeks apart and 18% were 2–4 weeks apart. The group at 2–4 weeks apart is suggestive of secondary infections, which are believed to be very rare in Campylobacter [Reference Blaser1]. Further analyses based on date of onset rather than reporting date are needed to quantify the incidence of secondary infections more precisely. It would also be important to ensure that this was not due to differential exposure to a foodstuff prior and post freezing.
Campylobacteriosis is more common in males than females up to about age 20 years [Reference Strachan9], but the male/female incidence ratio is about unity in older patients. This age-stratified change in the gender incidence ratio has been attributed to child-to-mother transmission [Reference Gillespie10]. In the dataset there were 11 groups which involved both an adult and a child aged <16 years with reporting dates more than 1 week apart. In seven of these, the child's infection was reported first, and six of the seven the adults were female. These trends are not statistically significant (binomial distribution P=0·06) and the one outbreak (outbreak no. 13) where MLST typing data are available shows different sequence types. Therefore further analyses based on larger datasets are needed for evaluating the contribution of child-to-mother transmission to the higher infection rates in adult females.
This study demonstrates that putative household outbreaks of Campylobacter in Scotland occurred at a similar incidence as previously found in Wales and Denmark, that household cases are much more common than general outbreaks and that ST strain composition can provide evidence that cases from such outbreaks are epidemiologically related.
ACKNOWLEDGEMENTS
The Food Standards Agency, Scotland funded this work. This publication made use of the Campylobacter jejuni multi-locus sequence typing website (http://pubmlst.org/campylobacter/) developed by Keith Jolley and Man-Suen Chan and sited at the University of Oxford.
DECLARATION OF INTEREST
None.





