INTRODUCTION
Melioidosis is an infectious disease caused by the soil organism Burkholderia pseudomallei. It is endemic in Southeast Asia and northern Australia and is most prevalent in tropical and subtropical areas between latitudes 20° N and 20° S of the equator. Within northern Australia it is particularly endemic in Townsville, North Queensland and cases occur mainly during periods of heavy rain during the wet season between January and May [Reference Malczewski1]. It is a clinically diverse infection with the most common sites of infection being the lungs, urinary tract, prostate, skin or soft tissue, spleen, and liver [Reference Cheng and Currie2]. Acute melioidosis commonly presents as a fulminant sepsis, often resulting in death within a few days of exposure [Reference Cheng and Currie2, Reference Chaowagul3]. Infection usually follows percutaneous inoculation, ingestion or inhalation of contaminated soil or water. Despite the initiation of intensive therapy, mortality remains at 21% in septic and non-septic patients with melioidosis in Australia [Reference Currie4].
Soil types have been proposed as being a factor in determining the distribution of the organism [Reference Strauss5, Reference Thomas, Forbes Faulkner and Parker6]. In one study, most isolates of B. pseudomallei were isolated from the clay layer of soil. The explanation for this was the electrostatic attraction of bacteria and water to clay particles. This ensured that a clay layer would retain water and bacteria which would normally be gravitationally pulled down to the water table. It was suggested that during the wet season, the water table rises closer to the surface and carries bacteria with it [Reference Thomas, Forbes Faulkner and Parker6]. In addition, environmental factors such as temperature, humidity and consistent rainfall play a critical role in the ability to recover the organism from the environment [Reference Strauss5, Reference Thomas, Forbes Faulkner and Parker6].
Within North Queensland, the city of Townsville and its suburbs are overrepresented in cases of melioidosis [Reference Malczewski1]. Between 1996 and 2002, there were 107 cases of culture-confirmed cases of melioidosis in the state of Queensland. Of these 88 (82%) were in Townsville and its surrounding regions (Tropical Population Health Unit, Cairns). This discrepancy is not explained by differences in population distribution or rainfall. The population of Townsville is about 150 000 of which around 9% are indigenous. This population distribution is not greatly different to that of another city in the region which has a significantly lower incidence of the disease. Paradoxically, Townsville with its high incidence of disease has lower annual rainfall than neighbouring Cairns.
A number of studies have attempted to link environmental isolates directly with clinical disease. Apart from outbreaks linked to potable water [Reference Inglis7, Reference Currie8], the results have not been promising [Reference Vesaratchavest9–Reference Dance11]. Within the Townsville region specifically, attempts to isolate the organism from soil around the suburban homes of patients with melioidosis in North Queensland were unsuccessful [Reference Inglis12]. This may have been due to the inappropriate site of soil sampling or the presence of viable but non-culturable organisms. There has also been interest in the interaction between B. pseudomallei and Acanthamoeba spp. [Reference Inglis13]. Although other Burkholderia spp. have been associated with the rhizospheres of specific plants, this has yet to be demonstrated with B. pseudomallei.
Anecdotal evidence suggested that disease occurrence in Townsville showed suburb specific clustering. The aim of this pilot study was to analyse the spatial distribution, using residential addresses of people with culture-confirmed melioidosis, to quantify purported disease clusters. A geographic information system (GIS)-based analysis was used to determine whether disease distribution is spatially and environmentally controlled. This analysis provides a framework from which hypotheses addressing the relationship between environmental controls (such as geology, soil and drainage) on disease acquisition can be formulated and tested.
METHODS
Subjects
Our melioidosis database comprises all culture-confirmed melioidosis cases presenting at The Townsville Hospital between 1996 and 2008. This was obtained from microbiology records held in a database. Addresses at time of presentation were ascertained from the database and those with Queensland postcodes 4810-4816 (in or around Townsville) were included in this study. Ethical approval was received from the Townsville Health Services District Human Ethics Committee.
GIS spatial analysis
The spatial attributes included in the analysis were geology, soil type, elevation (topography), drainage and cadastral divisions for the Townsville region. Vector digital data for geology is based on the ‘Townsville’ – 8259, 1:1 00 000 scale map sheet from Queensland Mapping Data (GEOLDATA), 2004 version, using the GDA94 Datum (Queensland Department of Natural Resources, Mines and Energy, Natural Resources Information Management). A vector digital version of the 1:1 00 000 soil map of Townsville [Reference Murtha14, Reference Murtha15] was obtained from the Queensland Department of Natural Resources and Water. Topographic data at 1:25 000 (5-m contours) (Queensland Natural Resources and Water, 2008) were used to develop the digital elevation model. Cadastral data for the Townsville region were obtained from the Queensland Department of Natural Resources and Water as at May 2008. ArcMap 9.3 (ESRI Inc., USA) GIS package was used for spatial analysis.
Case-distribution spatial analysis
Mapped point patterns of cases were analysed using Ripley's K function [Reference Ripley16–Reference Haase18] which allows for testing of variance of point-to-point distances in an empirical distribution against Poisson expectations. The method also allows for comparison of the observed distribution (melioidosis cases) to the distribution of the underlying population. This comparison tests for underlying population density control on case distribution.
We calculated Ripley's K(t) and L(t) (transformed K; see [Reference Haase18]) for distances up to 25 km at 500-m intervals (t). Upper and lower limits of significance were determined according to [Reference Haase18], using 30 randomly generated plot distributions of the same dimensions as the melioidosis cases. To allow for edge effects [Reference Haase18] we did not analyse the outer 500 m of the study area.
RESULTS
A total of 65 cases of culture-confirmed melioidosis occurred in subjects with recorded residential postcodes between 4810 and 4816, over the period 1996–2008. Residential addresses were available for all of these.
Ripley's K function analysis of both the underlying population distribution and the case distribution were compared against a simulated random distribution (Fig. 1). An observed L(t) value that exceeds the upper confidence limit for a random distribution indicates a statistically significant clustered pattern for that distance. Figure 1 shows that both the background population and the case distributions are significantly clustered relative to a random distribution. Comparison of L(t) values for the cases against the population shows higher L(t) values for the case distribution at distances less than ∼6 km and again at distances greater than ∼21 km. Therefore, at distances from 0·5 to ∼6 km, and ∼21–25 km cases are more clustered than the underlying population distribution.
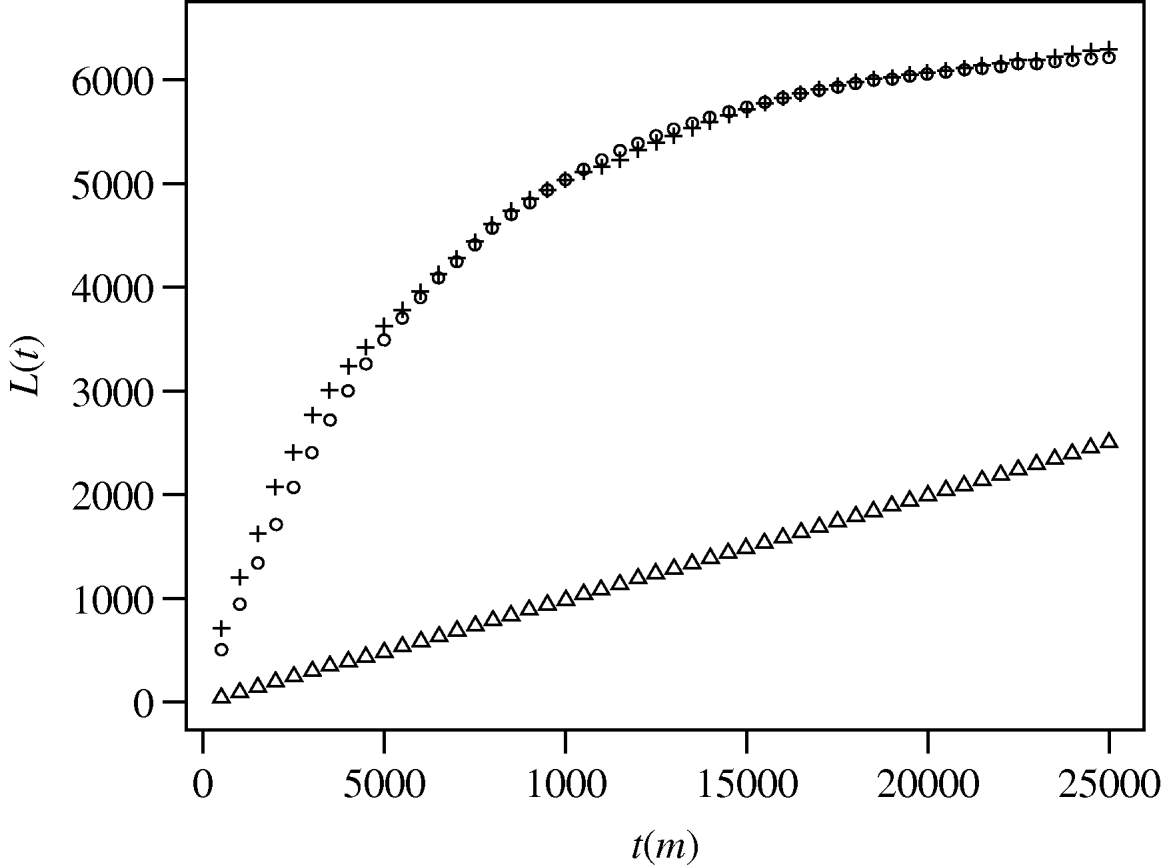
Fig. 1. Results of spatial pattern analysis of the distribution of melioidosis cases (+) and the Townsville population (○; point population distribution was determined by combining Australian Bureau of Statistics census data with cadastral lot distribution. Points were generated for each lot equal to a calculated lot population). For each 500-m interval the function K(t) was calculated and transformed to L(t) [Reference Haase18] and plotted against t. Triangles (▵) show the upper limit of the 95% confidence envelope for simulated spatial randomness (the lower limit is only slightly offset beneath the upper limit).
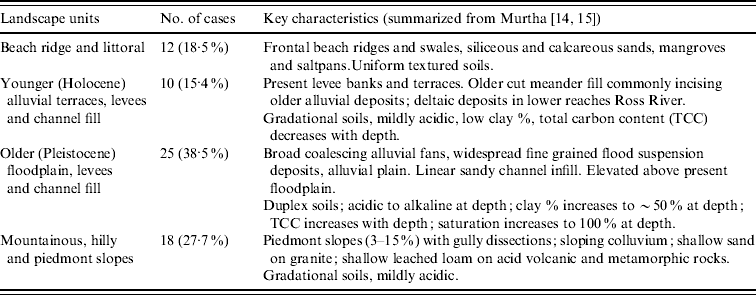
Of the 65 cases mapped, most occurred in two groups; 38·5% were found on Pleistocene alluvial terraces and fans and 27·7% were found around the base of hilly or mountainous terrain, in particular Castle Hill (Fig. 2). Two smaller cohorts associated with (a) younger (Holocene) alluvium channels and levees and (b) Holocene beach ridge and saltpans were identified (Table 1). Soils of the Pleistocene alluvial terraces and fans are mostly Sodosols [Reference Isbell19], which have an abrupt texture contrast between a slightly acidic sandy or silty loam topsoil (15–25 cm thick) and an alkaline sodic yellowish clay subsoil [Reference Murtha14, Reference Murtha15]. These soils are poorly drained due to the impermeable subsoil clay, so the topsoil becomes waterlogged following periods of heavy rainfall. The cases on Pleistocene alluvial terraces and fans tended to occur near the edges of that landscape unit, near to the boundary with younger alluvium. The collection of cases around the base of Castle Hill occurs on Kandosols [Reference Isbell19], which have grey-brown loamy sand to sandy loam topsoils overlying red or yellowish red sandy clay loam to sandy clay subsoils [Reference Murtha14, Reference Murtha15]. They are better drained than the Sodosols, but their position at the base of Castle Hill means they receive intense runoff following periods of heavy rainfall and therefore also become waterlogged for short periods. The remaining 21 cases were on better drained soils associated with recent alluvium, colluvium or beach ridges. There was a distinct absence of cases on the well-drained soils of the recent alluvial terraces and fans along the Ross River (Fig. 2).

Fig. 2. Distribution of melioidosis cases (1996–2008) defined by residential addresses in the Townsville region. Study area shown includes urban and semi-rural lots. Southern boundary is defined by 40-m topographic contour, above which residential development is absent. (a) Case distribution shown against landscape units defined by soil associations [Reference Murtha14, Reference Murtha15] (see Table 1). (b) Case distribution shown against property boundaries. (c) Case distribution shown against topography of Townsville coastal plain.
Table 1. Landscape units associated with melioidosis cases in the Townsville region
DISCUSSION
An association between B. pseudomallei and particular soil properties in the North Queensland area was first reported in 1979 [Reference Thomas, Forbes Faulkner and Parker6]. However, the precise ecological niche of B. pseudomallei remains to be defined. Whereas soil type has been associated with the presence of the organism this has not been demonstrated with clinical disease. A recent study from northern Australia demonstrated that there was an association between environmental isolates and the presence of grasses, disturbed soil, acid pH, livestock usage and soil texture [Reference Kaestli20]. Work from an endemic area in Thailand, has confirmed that soil pH, depth of at least 30 , a moisture content of >10%, higher total nitrogen and oxygen demand, all predispose to finding the organism in soil [Reference Palasatien21].
As expected in most urban settings, the spatial distribution of the underlying Townsville population differs significantly from any random point distribution of the same area. Figure 1 shows significant clustering of the underlying population. The broadly parallel L(t) trend of cases vs. population is likewise expected, since case distribution must be dependent to some degree on population distribution. However, the divergence of L(t) trends at two scales (<6 km and >21 km) indicates greater case clustering than would be expected if cases were simply controlled by population density.
Whereas second-order spatial statistical analysis such as Ripley's K function is useful in identifying clustering in the case distribution, it does not provide information about geographic locations of clusters. Mapping the cases directly onto environmental attributes, in particular soil, within the GIS considers the geographic distribution of cases in the context of soil type.
This study relates the presence of clinical disease with soil type. It is acknowledged that place of residence need not necessarily equate to the place of acquisition of disease. Nevertheless, given that case distribution is clustered independent of population density, there is a relationship between clinical cases and soil/geological features as demonstrated in Figure 2. There appear to be two groups of cases, one on poorly drained soils on Pleistocene alluvium and the other around the base of Castle Hill. Whereas these areas are predominantly residential with disturbed soil, this does not differ from other residential areas in the region which do not feature clinical cases of melioidosis. In addition, livestock usage does not feature in the residential areas of high incidence, nor is disease common in semi-rural areas with associated livestock (Fig. 2b). The association of clinical disease with periods of heavy rainfall, particularly with cyclonic conditions, is well recognized [Reference Cheng and Currie2]. It is postulated that both groups are associated with soil wetness following periods of intense rainfall, with one group on poorly drained soils at the lower edges of poorly drained alluvial plains and the other group located where large amounts of runoff are received from the adjacent granitic hill. The association with soil wetness and drainage appears to hold for other towns in North Queensland as well. Cairns has a similar population to Townsville and a similar although wetter climate, but has a low incidence of the disease. Unlike Townsville, Cairns has predominantly well-drained soils (Podosols, Kandosols and Dermosols [Reference Murtha, Cannon and Smith22]. Similarly, Innisfail, which lies between Cairns and Townsville, has well-drained soils [Reference Murtha23] and low incidence of the disease, even after the intensely wet period associated with Cyclone Larry in 2005. As previously mentioned, attempts to isolate the organism from the soils around Townsville have been unsuccessful [Reference Inglis12]. All soils in Townsville, apart from the saline tidal wetlands along the coast, are dry for most of the year, so the organisms must survive this period. B. pseudomallei is known to be particularly resistant to desiccation [Reference Inglis and Sagripanti24].
One confounding factor that has not been addressed in this study is the distribution of people with risk factors for the acquisition of melioidosis. These have been well described in the region and include diabetes (42%), excess alcohol consumption (42%), chronic lung disease (26%), immunosuppressive drug use (12%), renal disease (11%), malignancy (7%) and autoimmune disease (5%) [Reference Malczewski1]. There is no suggestion that the areas highlighted in this study are overrepresented with people with these risk factors compared to other areas. On the contrary, more heavily populated areas in the suburbs seem to be spared the higher incidence of disease (Fig. 2b). Of note, six of the 12 cases associated with the beach ridge and littoral landscape unit derive from two nursing homes. Clearly both human risk and environmental factors interrelate to control distribution of this disease. The domestic water supply for the region described is no different from that of the rest of the city and surrounds. A previous study did not find any evidence of the organism in the water supply of Townsville [Reference Inglis12].
Our approach in the next phase of this research focuses on integrating geospatial parameters, field-based microbiological culture and molecular detection, and epidemiological analysis of the Townsville case database. We are undertaking a small-site epidemiological assessment of human health, demographic and socioeconomic factors. We will analyse temporal case distribution against climatic events, and individual risk factors such as ethnicity, socioeconomic status, and occupation. This will be correlated with potentially differing exposure environments. It is proposed to explore further the relationship between soil type and B. pseudomallei using more detailed soil sampling during the wet season, molecular detection and culture. Our field sampling programme will be informed by the ‘high incidence’ landscape units identified in this study. In addition an in-vitro model of soil type with simulated rainfall may provide useful information on B. pseudomallei response to simulated environmental conditions. We will also molecularly type isolates from the two areas described to determine clonality, if present. However, previous studies have demonstrated that clinical isolates of B. pseudomallei are genetically diverse [Reference Cheng25]. It is hoped that this multifaceted approach will provide a greater insight into the ecological niche of this important pathogen.
ACKNOWLEDGEMENTS
Martin Labadz and Stefan Loehr provided technical assistance with ArcGIS. Stefan Loehr undertook the Ripley's K analysis. This study is supported by a grant from The Townsville Hospital Private Practice Study, Research and Education Trust Fund.
DECLARATION OF INTEREST
None.







