Vitamin K refers to a group of compounds which can act as cofactors for the enzyme γ-glutamyl carboxylase. Its function is to facilitate post-translational conversion of specific glutamyl residues to γ-carboxyglutamyl residues in a number of proteins, particularly in several involved in the blood coagulation cascade. This enzyme also catalyses the carboxylation of osteocalcin in bone.
Phylloquinone, the major dietary form of vitamin K, is found predominantly in green, leafy vegetables. The bioavailability of purified phylloquinone has been reported to range from as low as 2 % to as high as 80 %(Reference Jones, Bluck and Wang1, Reference Shearer, Barkhan and Webster2). Other studies have reported the relative bioavailability of food-bound phylloquinone compared to that of a supplement, with the conclusion that the bioavailability of phylloquinone from vegetables is significantly less than that from purified phylloquinone(Reference Booth, Lichtenstein and Dallal3–Reference Garber, Binkley and Krueger5), though no specific values for bioavailability of phylloquinone from vegetables have been reported. To determine the bioavailability of phylloquinone from a green, leafy vegetable, we conducted a study in which volunteers consumed isotopically labelled kale, and provided multiple blood samples for several weeks following the kale ingestion. A compartmental model was constructed to calculate the bioavailability of phylloquinone from kale, and to determine body pool size and rates of elimination.
Experimental methods
Isotopic labelling of kale
The method by which the nutrients in kale (Brassica oleracea var. Acephala cv. Vates) were labelled with 13C has been described previously(Reference Kurilich, Britz and Clevidence6). Briefly, kale tissue was completely labelled with 13C by growing plants in a controlled environmental chamber with the only source of carbon being 13CO2. A sealed clear acrylic box (Plexiglas G; Rohm and Haas, Wilmington, DE, USA) with a separate base was placed inside the controlled environmental chamber. The base and top of the acrylic box were fitted together via a water trough to provide a leak-proof seal, thus preventing exchange of 13CO2 with atmospheric CO2. Plants which were transferred into the growth chamber as seedlings were grown in Nalgene bins placed inside the acrylic box. Nutrient solution was delivered through tubes attached to the base portion of the acrylic box. Growth conditions were maintained as follows: temperature of 24°C; humidity of 90 %, light with a 24 h photoperiod, 900 μmol/m2 per s of photosynthetically active radiation 400–700 nm, and the partial pressure of CO2 (13CO2, >99 % carbon-13) inside the chamber was maintained at 36 Pa.
Kale plants were harvested at leaf canopy closure (approximately 10 d). Roots and primary leaves were removed, and material for the feeding study was cleaned, weighed and blanched. The blanched kale was chopped into 1″ × 1″ pieces, mixed for batch uniformity and frozen at − 80°C until prepared for consumption.
Subjects, study design, diet and sample collection
Seven healthy, non-smoking volunteers (four males and three females) from the Beltsville, MD, area participated in the present study. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Johns Hopkins University Bloomberg School of Public Health Committee on Human Research. Written informed consent was obtained from all subjects. Subjects' characteristics (means and standard deviations) were 46 (sd 14) years of age, 71 (sd 8) kg body weight and BMI of 25 (sd 3) kg/m2.
Each subject consumed a single 400 g (three cups) serving of 13C-labelled kale. Before consumption, frozen kale was transferred to a refrigerator (6°C) to thaw for 12 h, and then warmed in a 900 W microwave oven for 2 min. We administered the kale to a single individual as a pilot test to ensure that the nutrients were elevated above the detection limit before proceeding with the remaining subjects. The kale was consumed with 30 g peanut oil (n 1, pilot subject) or 30 g safflower oil (n 6). The kale treatment provided 156 nmol phylloquinone (equivalent to 70 μg unlabelled phylloquinone). The peanut oil contained 0·2 μg phylloquinone per 30 g and the safflower oil contained 2·1 μg phylloquinone per 30 g, with both oils contributing minimal vitamin K compared with the kale treatment(7).
Subjects were provided a controlled diet for 1 week before kale ingestion and throughout the collection period. All foods were provided by the Beltsville Human Nutrition Research Center, and subjects were instructed to consume all foods and only foods provided by the Nutrition Center. The diet contained 15 % of energy from protein, 32 % from fat and the remainder from carbohydrate. The vitamin K content of the diet depended on the energy level of the subjects, and provided 119 μg vitamin K per 8·4 MJ (2000 kcal)(7). Vitamins and supplements were prohibited throughout the study. On the 13C-kale treatment day, lunch and dinner were provided at 5 and 10 h after the treatment.
Blood collection times were as follows: ten collections across the treatment day, two collections (AM and PM) on the following day, each morning for the next 3 d (fasting) and semi-weekly for the next 3 weeks (fasting). Blood samples were collected into vacutainers containing EDTA, and were centrifuged at 2560 g for 10 min. Plasma aliquots of 1 ml were stored in cryovials at − 80°C until analysis.
Reagents
Chloroform, ethanol, hexane, isopropanol, methanol and methyl tertiary butyl ether were purchased from Fisher Scientific (Pittsburgh, PA, USA). Phylloquinone, β-apo-8′-carotenal and butylated hydroxytoluene were purchased from Sigma Chemical Company (St Louis, MO, USA). 13CO2 (>99 % carbon-13) was purchased from Isotec, Inc. (Miamisburg, OH, USA).
Kale extraction
Kale was ground into a fine powder with liquid N2 using a mortar and pestle. Three-gram samples of powder were weighed into each of two 25 ml test-tubes for extraction, and 0·0178 μg of β-apo-8′-carotenal was added as an internal standard. Protein was precipitated with 9 ml of 0·1 % butylated hydroxytoluene in ethanol and vortex-mixed for 2 min. Hexane–toluene (5:4, v/v, 9 ml) was added and the samples were vortex-mixed and then placed in a 70°C water-bath for 10 min. After the addition of 180 μl of 80 % KOH, the samples were vortex-mixed and returned to the water-bath for 15 min, with vortex-mixing for 8 min. The samples were then placed on ice, and 3 ml of double-distilled water were added before vortex-mixing again. After sample centrifugation at 1140 g for 10 min, the organic layer was transferred to a separate test-tube. The extraction was repeated two additional times with 6 ml hexane–toluene (10:8, v/v). A 100 μl aliquot was taken from the combined organic layer, dried under N2 and reconstituted in 200 μl of methyl tertiary butyl ether–methanol (1:1, v/v) before injection onto the liquid chromatography–MS apparatus.
Plasma extraction
The plasma analysis procedure used was the one optimised for simultaneous measurement of carotenoids, retinol and phylloquinone(Reference Kurilich, Britz and Clevidence6). Phylloquinone and 13C31-phylloquinone were extracted from 0·5 ml of plasma. Plasma was thawed, and β-apo-8′-carotenal (50 μl of 0·356 μg/ml) was added as an internal standard immediately before the addition of 0·5 ml of ethanol for precipitation of protein. Samples were extracted twice with 1·5 ml hexane. The combined hexane extracts were dried under N2, and reconstituted in 200 μl of methyl tertiary butyl ether–methanol (1:1, v/v) before injection onto the liquid chromatography–MS apparatus.
Liquid chromatography–MS conditions
An Agilent Technologies (Palo Alto, CA, USA) 1100 series liquid chromatography instrument was used. The instrument included a cooled autosampler, automatic solvent degasser, binary pump, cooled column compartment, a G1315A diode array detector and a G1946A mass spec detector with an atmospheric pressure chemical ionisation source. An YMC C30 column (3 μm, 250 × 4·6 mm) and C30 guard cartridge were used. The mass spec detector source conditions were as follows: spray chamber gas temperature set at 350°C, vaporiser temperature set at 400°C, nitrogen nebuliser pressure set at 45 psig (310 kPa) and corona current set at 5 μA. Initially, the VCap was set to 3800 V and the flow rate of the drying gas (nitrogen) was set to 7 l/min; at 7·5 min, the VCap changed to 3600 V and flow rate of the drying gas to 6 l/min. Selected ion monitoring was used for the detection of protonated unlabelled phylloquinone and 13C31-phylloquinone (m/z 451 and 482) and internal standard β-apo-8′-carotenal (m/z 417). Data were collected using the Agilent Chemstation software. Molar concentrations of labelled and unlabelled phylloquinone were calculated based on a response curve for β-apo-8′-carotenal v. unlabelled phylloquinone. The limit of detection and limit of quantification for phylloquinone were 3 and 10 fmol, respectively.
The solvent system consisted of 1 mm-ammonium acetate in methanol (solvent A) and methyl tertiary butyl ether (solvent B). A solvent gradient was used in which the mobile phase consisted of 15 % solvent B at 0 min, which increased linearly to 30 % solvent B at 12 min. The mobile phase was held at 30 % solvent B until 18 min. Then, solvent B was increased to 100 % and held until 23 min, and was then decreased back to 15 % for 10 min for column re-equilibration. The injection volume was 50 μl, and the flow rate was 1 ml/min.
Calculations
Compartmental modelling was performed using the WinSAAM software package. A three-compartment catenary model was constructed with compartments for the gastrointestinal tract (compartment 1), the plasma (compartment 2) and a body tissue pool (compartment 3), and initial transfer parameters were estimated based on the features of the plasma phylloquinone concentration–time curve. Parameters were then adjusted in physiologically compatible ways to improve the fit of the model prediction to observed data values. Once the model and parameters provided good visual fits to the 13C-phylloquinone data, a least-squares procedure was used to minimise the difference between model prediction and observed data. Each subject was modelled individually. The Extended Multiple Studies Analysis feature in WinSAAM was used to analyse the group of subjects as a whole.
Results
Six of the subjects showed significant amounts of labelled phylloquinone in plasma, though one subject's plasma was not consistently enriched above the detection limit, and this subject's baseline plasma phylloquinone level was the lowest in the group. Of the subjects who responded to the kale, labelled phylloquinone was first detected in the plasma at 3 h after kale ingestion (Fig. 1) and it was observed throughout the 28 d sampling period for three subjects, and for shorter periods for the other three subjects (through day 24 for one subject, through day 22 for one subject and through day 7 for one subject) (Fig. 1). The time of peak plasma concentration ranged between 6 and 10 h. Mean peak 13C-phylloquinone concentration in plasma was 2·12 nmol/l (range 0·37–5·1 nmol/l, n 6). In all cases, there was a sharp rise in plasma 13C-phylloquinone following kale ingestion, followed by a sharp decline, and then maintenance of plasma 13C-phylloquinone at a low level thereafter.

Fig. 1 Mean plasma 13C-phylloquinone concentration (nmol/l) as a function of time after ingestion of labelled kale. Inset shows the same data for the first 72 h of sampling. Data represent mean values with their standard errors for the six subjects whose plasma values rose above the quantification limit.
A three-compartment catenary model was used to analyse the pharmacokinetics of the plasma 13C-phylloquinone for the six subjects whose plasma levels rose significantly. This model provided a good prediction of plasma phylloquinone levels for each subject, with good statistical certainty on fractional transfer coefficients between compartments (all CV values for transfer coefficients were less than 20 %). The model prediction compared with experimentally measured plasma 13C-phylloquinone values for a representative subject is shown in Fig. 2. Pharmacokinetic analysis provided values for bioavailability, mass of vitamin K absorbed from kale and half-time values (Table 1). The bioavailability of phylloquinone from kale was found to be on average 4·7 %, and it ranged from 1 to 14 %. Thus, on average, 7·2 nmol (3·2 μg) of the 156 nmol phylloquinone ingested in the labelled kale were absorbed into the systemic circulation.
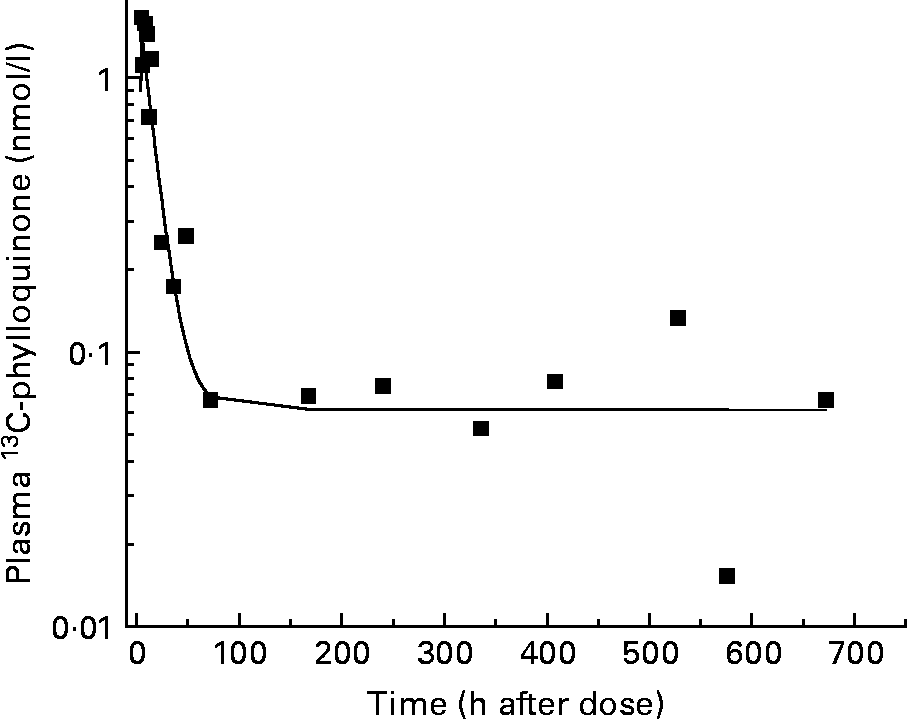
Fig. 2 Model-predicted (—) and observed (■) plasma 13C-phylloquinone concentration for a representative subject. The model prediction is in good agreement with observed data.
Table 1 Results from pharmacokinetic modelling*
(Mean values and standard deviations)
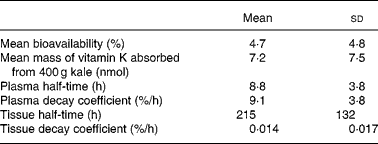
* Plasma 13C-phylloquinone concentrations of six subjects rose above the quantification limit.
Using the model, the vitamin K utilisation rate and tissue storage pool were calculated for a man weighing 86 kg, which is the mean body weight for an adult male in the United States(Reference McDowell, Fryar and Hirsch8), and a woman weighing 74 kg, which is the mean body weight for an adult female in the United States(Reference McDowell, Fryar and Hirsch8), with plasma phylloquinone levels of 1·43 and 1·47 nmol, respectively(Reference Booth, Tucker and McKeown9). The tissue storage pools for the representative man and woman were found to be 103 nmol (46 μg) and 91 nmol (41 μg), respectively.
Discussion
A number of studies previously have used purified, isotopically labelled phylloquinone to investigate vitamin K kinetics in human subjects. Phylloquinone has been labelled with 3H(Reference Shearer, Barkhan and Webster2, Reference Shearer, Mallinson and Webster10–Reference Bjornsson, Meffin and Swezey14), carbon-13(Reference Jones, Bluck and Wang1, Reference Jones, Bluck and Wang15) and 2H(Reference Jones, Bluck and Wang1). Oral doses have ranged from 3 μg (3 times/d for 3 d) to 1 mg (as a single bolus dose)(Reference Jones, Bluck and Wang1, Reference Shearer, Barkhan and Webster2, Reference Shearer, McBurney and Barkhan12, Reference Jones, Bluck and Wang15). Other studies have involved intravenous injections of labelled vitamin K(Reference Jones, Bluck and Wang1, Reference Olson, Chao and Graham13, Reference Bjornsson, Meffin and Swezey14). Sampling times after dosing have ranged from 7 h to 6 d(Reference Jones, Bluck and Wang1, Reference Shearer, Barkhan and Webster2, Reference Shearer, Mallinson and Webster10–Reference Shearer, McBurney and Barkhan12, Reference Bjornsson, Meffin and Swezey14, Reference Jones, Bluck and Wang15). In addition, two studies have involved labelled phylloquinone delivered in a plant matrix. Dolnikowski et al. (Reference Dolnikowski, Sun and Grusak16) showed feasibility by administering broccoli labelled with 2H to a single male subject and collecting blood for 24 h. Erkkila et al. (Reference Erkkila, Lichtenstein and Dolnikowski17) labelled collard greens with 2H, fed the product to volunteers and measured labelled phylloquinone in fractionated plasma for 72 h after ingestion of the dose.
The previous studies with labelled phylloquinone, as well as some with unlabelled phylloquinone, have provided limited information about phylloquinone bioavailability. These studies have generally involved evaluation of the area under the plasma concentration–time curve after the volunteers ingested vitamin K in the form of vegetables, supplements or fortified oil(Reference Booth, Lichtenstein and Dallal3–Reference Garber, Binkley and Krueger5, Reference Booth, O'Brien-Morse and Dallal18). Overall, these studies have provided bioavailability estimates of purified vitamin K ranging widely from 2 to 80 %. The dual-isotope method for phylloquinone bioavailability published by Jones et al. (Reference Jones, Bluck and Wang1) suggested that the bioavailability of purified phylloquinone was 13 (sd 9) %. Previous studies have also suggested that the food matrix inhibits absorption of vitamin K, as plasma response after ingestion of vitamin K from plant-based foods has been lower than that for purified vitamin K(Reference Booth, Lichtenstein and Dallal3–Reference Garber, Binkley and Krueger5, Reference Schurgers and Vermeer19). Jones et al. (Reference Jones, Bluck and Wang15) demonstrated that different meal components ingested with phylloquinone can significantly impact bioavailability. Gijsbers et al. (Reference Gijsbers, Jie and Vermeer4) compared the bioavailability of phylloquinone from spinach to that from a pharmaceutical preparation. By comparing the plasma phylloquinone response after each treatment, they concluded that relative bioavailability of spinach was 4–12 % of that of the supplement. Since the reports of phylloquinone bioavailability have been widely ranging, this could correspond to a bioavailability from spinach of 0·1–10 %. The present study suggests a mean bioavailability of phylloquinone from kale of 4·7 %, with a range of 1–14 %.
The inter-individual variability in phylloquinone bioavailability reported here is slightly higher than that found in a previous study of purified phylloquinone(Reference Jones, Bluck and Wang1). Jones et al. (Reference Jones, Bluck and Wang1) reported a larger range of bioavailability (2–26 %) than that we observed (1–14 %), though the CV for the earlier study was 69 %, while the CV found in the present study was 100 %. The study of Jones et al. (Reference Jones, Bluck and Wang1) involved a larger number of subjects, which would be expected to reduce the CV compared to that of the present study involving fewer volunteers. In addition, differences in the ability to digest the plant matrix would be expected to increase the variability in the present study compared to a study of purified phylloquinone. Still, the inter-individual variability of the two studies is similar.
Several previous studies have estimated vitamin K tissue pool size by extrapolating the terminal decay curve back to its intercept. Jones et al. (Reference Jones, Bluck and Wang1) predicted a total body pool size of 5·1 nmol (2·3 μg), while Olson et al. (Reference Olson, Chao and Graham13) predicted an average pool size of 194 nmol (87·6 μg) for moderate vitamin K intake levels and 99 nmol (44·7 μg) after several weeks of low vitamin K intake. We used compartmental modelling to simulate pool sizes, given the plasma phylloquinone concentrations found in the older population(Reference Booth, Tucker and McKeown9) and the mean body weights for men and women(Reference McDowell, Fryar and Hirsch8) in the US. Our calculations suggest that an average man and woman would have 103 nmol (46 μg) and 91 nmol (41 mg) of stored phylloquinone, respectively. These are in accordance with the values estimated by Olson et al. (Reference Olson, Chao and Graham13).
Several of the previous studies have reported half-times for vitamin K disappearance, suggesting two phases of decay: one with a faster half-time, ranging from 0·21 to 1·0 h, and the other with a slower half-time, ranging from 1·8 to 3·4 h(Reference Jones, Bluck and Wang1, Reference Shearer, Mallinson and Webster11, Reference Shearer, McBurney and Barkhan12, Reference Bjornsson, Meffin and Swezey14). These studies were fairly short in duration, ranging from 6 to 10 h; thus, only early kinetic behaviour could be monitored. Olson et al. (Reference Olson, Chao and Graham13) conducted a kinetic study of longer duration (6 d) to find substantially longer half-times of 1·0 and 27·6 h, with the latter half-time being similar to the 22·8 h reported by Erkkila et al. (Reference Erkkila, Lichtenstein and Dolnikowski17) for a study of labelled collard greens. The present study extended measurements for several weeks after kale ingestion, allowing us to detect a significantly slower elimination curve than the curve that had been observed previously and producing a half-time for tissue elimination of 215 h. While this is substantially longer than the values reported for the shorter duration studies of plasma kinetics, it is substantially shorter than what would be predicted from analysis of tissue pools predicted in Olson's study(Reference Olson, Chao and Graham13). Olson et al. (Reference Olson, Chao and Graham13) conducted the kinetic study under conditions of high and low phylloquinone intake. From the plasma decay curves, pool sizes were calculated by extrapolating the curve back to its intercept for moderate vitamin K intake and after several weeks of low vitamin K intake. Olson et al. (Reference Olson, Chao and Graham13) derived an equation for vitamin K pool size based on phylloquinone plasma levels. Before depletion, Olson reported a plasma phylloquinone concentration of 1·94 nmol/l on average, which would correspond to a body pool of 99 μg for a man weighing 86 kg, the average body weight of an adult male in the United States(Reference McDowell, Fryar and Hirsch8). After depletion, plasma concentration dropped to 1·05 nmol/l, corresponding to a pool size of 21·5 μg. The pool was depleted 78 % over the 66 d. In general, 75 % depletion corresponds to two half-times. Thus, if two half-times occurred over approximately 66 d, then one half-time is 33 d or 792 h.
The model presented here describes the kinetics of vitamin K in the form of phylloquinone. The primary circulating form of vitamin K in blood is phylloquinone. Shearer et al. (Reference Shearer, Barkhan and Webster2) found that 80 % of radioactivity in plasma after consumption of an oral dose of tritiated phylloquinone was in the form of phylloquinone at the peak. Olson et al. (Reference Olson, Chao and Graham13) reported that 2 h after administering tritiated phylloquinone to volunteers, 90 % of the radioactivity in the plasma was associated with authentic phylloquinone. In the model, conversion of phylloquinone to metabolites would be captured in the calculation of elimination of phylloquinone from plasma. However, the kinetics of specific metabolites is not included in this analysis.
In conclusion, the administration of 13C-phylloquinone to adult volunteers and subsequent compartmental analysis have revealed new information about bioavailability, pool size and elimination of vitamin K. Bioavailability of vitamin K from kale is low, pool sizes may be larger than those previously estimated and measurement of terminal elimination rates requires sampling times over several weeks.
Acknowledgements
The authors' contributions are as follows: J. A. N. and B. A. C. designed the study; S. J. B. produced the labelled plant material; A. C. K. developed and conducted laboratory analysis; J. A. N. and D. J. B. interpreted data; and J. A. N. wrote the manuscript. The present work was funded by the U.S. Department of Agriculture. There are no conflicts of interest related to the present work. The present research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.







