It is well established that nutrient intake during pregnancy plays a significant role in maternal and child health(Reference Scholl1). Hackney in the East End of London has a substantial immigrant population with high levels of social and economic deprivation, mirroring many large cities throughout the developed world. Deprivation is associated with poor birth outcomes such as low birth weight (LBW), preterm birth and intra-uterine growth restriction(Reference Kramer, Seguin and Lydon2). Previous research here has demonstrated poor micronutrient intakes and status, including Fe, folate and thiamin, which have been associated with such adverse pregnancy outcomes(Reference Doyle and Rees3–Reference Rees, Doyle and Srivastava5).
The most recent Cochrane review(Reference Haider and Bhutta6) states that there is insufficient evidence to suggest that multiple-micronutrient supplementation is any more effective at improving birth outcome than Fe and folic acid supplements alone. However, the authors concede that further research is required due to a lack of well-controlled studies in this area. Furthermore, many studies are now over 20 years old and more recent studies have been in developing countries, making it difficult to extrapolate the results to industrialised populations.
An observational study of multiple-micronutrient supplement use in early pregnancy amongst low-income women (n 1430) in the USA found supplement users had a twofold reduced risk of preterm birth and LBW; beginning supplement use in the first trimester led to a greater reduction in risk compared with the second trimester(Reference Scholl, Hediger and Bendich7). However, this was not a randomised, controlled trial, and women choosing to use supplements may have had an intrinsically lower risk of poor birth outcome compared with non-users. A small randomised controlled trial in France (n 65) showed that women receiving multiple-micronutrient supplements from about 14 weeks of gestation had improved micronutrient status and gave birth to infants with a mean birth weight 10 % higher than those receiving placebo(Reference Hininger, Favier and Arnaud8). Although the French results are promising, the results from such a small sample size require corroboration.
This present study builds on previous research and is the first double-blind, randomised and controlled trial of multiple-micronutrient supplementation from the first trimester of pregnancy in a socially deprived, multi-ethnic population within a developed country. We assessed the effect of such supplementation on maternal nutrient status, namely Fe, folate, thiamin and vitamin D, and infant birth weight and gestational age at birth.
Subjects and methods
Participants
Between June 2002 and May 2004 women were recruited at their booking (first) appointment from the Homerton Hospital antenatal clinic and two community clinics in the borough of Hackney in East London, as previously described(Reference Brough, Rees and Crawford9). Volunteers were aged 16 years or older with a singleton pregnancy. Exclusion criteria included a gestation of greater than 13 weeks of gestation, chronic disease or use of micronutrient supplements (excluding folic acid and Fe). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the East London and the City Health Authority research ethics committee. Informed, written consent was obtained from all participants; non-English speaking women were only recruited if a suitable advocate was available.
Intervention
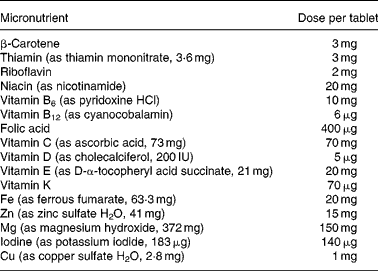
Participants were randomised to receive either multiple-micronutrient supplements (Table 1), known as Pregnacare, or a visually identical placebo comprising starch with an iron oxide coating. All tablets were provided by Vitabiotics (London, UK) and packaged to allow double blinding. Only Vitabiotics knew the code and it was not broken until statistical analysis had been completed. Each participant was instructed to take one tablet daily with food and plenty of water from recruitment until her baby was delivered. Women not using folic acid were also given 400 μg folic acid to take daily until 12 weeks of gestation.
Table 1 Micronutrient composition of treatment tablets
Anthropometric, demographic and ethnic data
At booking, height was ascertained to the nearest 1 cm and weight was measured, without shoes in light clothing, to the nearest 1 kg. Obstetric and medical information was available from hospital notes. Estimated date of delivery and gestational age at booking were determined by a qualified sonographer using an ultrasound scan. Participants reported over fifty different ethnicities; to aid data analysis these were reduced to five ethnic groups: African, Asian, Caucasian, West Indian and Other.
Follow-up and compliance
Participants were followed up at routine appointments at 20, 26 and 34 weeks of gestation. Subjects were questioned about frequency of supplement use and more tablets were provided as required. If supplement use had ceased, information was obtained regarding length and frequency of use together with reason for cessation.
Nutrient status
Hb and packed cell volume (PCV) were routinely determined on EDTA-stabilised whole blood samples, using a Coulter STKS analyser (Beckman Coulter, Brea, CA, USA), thus data were available for the majority of participants at recruitment and 26-week and 34-week visits. Serum ferritin and erythrocyte folate were measured using a DPC Immulite 2000 analyser (DPC, Los Angeles, CA, USA), a fully automated immunoassay analyser. Measures were made on the majority of the samples at booking, but only for compliant participants at 26-week and 34-week visits.
An additional blood sample was taken from compliant participants; lithium-heparin stabilised whole blood was spun and packed erythrocytes and plasma were stored at − 70°C. Thiamin diphosphate (TDP) in the erythrocytes was determined directly using HPLC coupled with a spectrofluorimeter and reagent kit (Chromsystems, Manchester, UK); TDP in whole blood was then calculated. TDP was measured in a subset of samples at booking and 34 weeks of gestation only. Plasma was used to measure 25-hydroxyvitamin D3 directly using HPLC with UV detection; internal standards from Chromsystems (Manchester) were used for HPLC calibration. Vitamin D was assessed at booking, and at 26-week and 34-week appointments. The season of sample collection was defined as summer (April to September) and winter (October to March). For technical reasons we were unable to analyse all samples for vitamin D (especially at 26 weeks); hence the low numbers of data. All analysis was performed at the Homerton Hospital pathology laboratory (London, UK), except for TDP which was analysed at Epsom Hospital (Epsom, Surrey, UK).
Obstetric data
Obstetric and medical information was available from hospital notes. Immediately after birth, infant weight was determined to the nearest 10 g using weighing scales and the infant's head circumference was measured using a measuring tape to the nearest 0·5 cm. All measures were determined by a midwife. Infants weighing less than 2500 g at birth were defined as LBW. Preterm birth was defined as infants born before 37 weeks of gestation (based on ultrasound scan). Centiles were calculated based on birth weight for gestational age using sex-specific, British 1990 growth reference data provided by the Child Growth Foundation (London, UK). Infants below the 10th centile of weight for gestational age were defined as small for gestational age (SGA).
Statistical analysis
Statistical analyses were carried out using SPSS 15.0 for Windows (SPSS, Inc., Chicago, IL, USA). Normality of data was assessed using the Kolmogorov–Smirnoff test and visual inspection of Q-Q plots. Normally distributed data are presented as means with standard deviations; non-normally distributed data are expressed as medians with interquartile ranges. Differences between treatment groups were assessed using independent t tests (two-tailed; parametric) or the Mann–Whitney U test (two-tailed; non-parametric). Categorical associations were examined using χ2. Intention-to-treat analysis was used for measures of Hb and PCV. However, measures of serum ferritin, erythrocyte folate, thiamin and 25-hydroxyvitamin D3 were only available for compliant participants at 26 and 34 weeks of gestation; thus analysis of these represents a subset. Spearman's rank correlation coefficient was used to determine associations between two numerical variables; potential correlations were verified using scatter plots.
Nutrient data which were not normally distributed were transformed logarithmically before analysis to approximate normal distribution. The data were analysed for an effect of supplement and time by repeated-measures ANOVA using the general linear model. Post hoc comparisons with Bonferroni's correction were used to test for specific comparisons between time-points and between the two groups (supplement and placebo) at each time-point.
Results
Participants
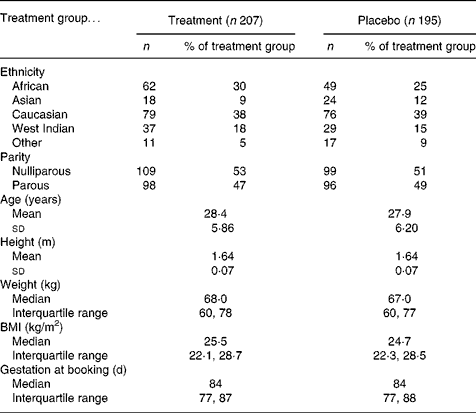
Of the 2385 women identified as suitable based on general practitioner referral letter, 402 agreed to participate (Fig. 1) and 342 refused to participate. The remainder either did not present for the first antenatal appointment (n 365), were missed when the researcher was with another participant (n 216), were found not to meet the inclusion criteria (n 807) or were non-English speaking and no advocate was available (n 253). Participants were aged between 16 and 42 years with a gestational age at recruitment between 35 and 125 d (Table 2). Subsequent to recruitment, thirteen participants were found by ultrasound scan to have a gestation of greater than 13 weeks (13+1 to 17+6); they remained in the study and the final analysis, as their inclusion afforded little difference in the nutrient data. Two women were carrying twins and were removed from the study. Of the recruits, 207 (51 %) were allocated to the treatment group and 195 (49 %) to the placebo group. There were no significant differences in age, height, weight, BMI or parity regarding treatment group allocation.

Fig. 1 Flow chart showing treatment allocation and participant compliance.
Table 2 Description of 402 participants at booking by treatment group
Compliance
Only 39 % of participants remained compliant for the duration of the study. Reasons for non-compliance were many and varied (Table 3). Motivation was a substantial problem; 20 % of non-compliers decided that they no longer wanted to take part in the study and 17 % either forgot to take their tablets or lost them. Of non-compliers, 5 % moved away, reflecting the highly mobile population and 14 % of non-compliers stopped for medical reasons such as a high-risk pregnancy. Despite the study being widely advertised amongst local health professionals, 9 % of non-compliers were advised to cease the supplements for reasons which did not preclude them, such as nausea, low Hb or prescription of Fe supplements.
Table 3 Reasons for non-completion of the study according to treatment group

Participants were regarded as compliers if they reported taking five or more tablets per week until the 34-week appointment (median 240 (interquartile range 238, 242) d) or 26-week appointment (median 184 (interquartile range 182, 187) d) if delivery was before 34 weeks of gestation. Participants receiving the treatment were more likely to be compliers and those receiving placebo were less likely to be compliers (P = 0·022; χ2 test). Compliers were on average significantly older (29·1 v. 27·6 years; P = 0·016; t test) and had a greater median gestational age at booking (85 v. 83 d; P = 0·002, Mann–Whitney U test) than non-compliers; however, there were no differences at recruitment regarding nutritional status for compliers compared with non-compliers.
Infant birth data
Data were available for 353 live-born singleton infants. Using intention-to-treat analysis there were no significant differences between treatment and placebo infants regarding mean birth weight, gestational age at birth or number of infants defined as SGA (Table 4). However, considering only compliant mothers, the women in the placebo group were significantly more likely to have an SGA infant compared with those in the treatment group (P = 0·042; χ2). On removing those participants who were recruited at greater than 13 weeks of gestation the significance no longer remained. However, it could be argued that the 13-week recruitment criterion is an arbitrary cut-off and that the supplement may also be beneficial for women just over 13 weeks of gestation.
Table 4 Description of birth outcome by treatment group
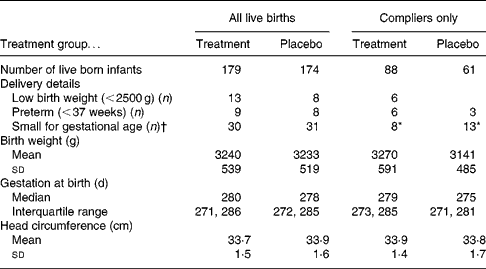
* P = 0·042 (χ2).
† Small for gestational age defined as < 10th centile birth weight for gestational age.
Nutrient status
Using intention-to-treat analysis, repeated-measures ANOVA showed a significant overall difference in Hb concentrations (Table 5) by treatment (F(1, 285) = 207·55; P = 0·033) and gestation (F(2, 570) = 4·59; P < 0·001) and an interaction between treatment and gestation (F(2, 570) = 4·89; P = 0·012). Post hoc analysis showed that Hb concentrations were significantly lower in the placebo group compared with the treatment group at 26 weeks of gestation (P = 0·041) and 34 weeks of gestation (P = 0·003). There was also an overall difference in PCV concentrations with gestation (F(2, 570) = 169·54; P < 0·001) and treatment (F(1, 285) = 5·04; P = 0·025), but no interaction between gestation and treatment (F(2, 570) = 2·29; P = 0·111). Post hoc analysis showed that PCV concentrations were significantly lower in the placebo group compared with the treatment group at 26 weeks of gestation (P = 0·011) and 34 weeks of gestation (P = 0·014).
Table 5 Maternal micronutrient status at recruitment, 26 and 34 weeks of gestation according to treatment group

Data were analysed for an effect of gestation and treatment using repeated-measures ANOVA. Values were significantly different from those of the placebo group (post hoc comparisons with Bonferroni's correction): * P = 0·041, † P = 0·003, ‡ P = 0·011, § P = 0·014, ∥ P = 0·026, ¶ P < 0·001, ** P = 0·018.
†† Results expressed as median and interquartile range.
Analysis of a compliant subset showed an overall difference in serum ferritin concentrations with gestation (F(2, 100) = 80·15; P < 0·001) and an interaction with treatment (F(2, 100) = 5·38; P = 0·008) but not for treatment alone (F(1, 50) = 0·1; P = 0·910). There was an overall difference in erythrocyte folate concentrations with gestation (F(2, 198) = 22·13; P < 0·001) and treatment (F(1, 99) = 37·84; P < 0·001) and an interaction between treatment and gestation (F(2, 198) = 17·92; P < 0·001). Post hoc analysis showed that erythrocyte folate concentrations were significantly lower in the placebo group compared with the treatment group at booking (P = 0·026), 26 weeks of gestation (P < 0·001) and 34 weeks of gestation (P < 0·001). Also there was an overall difference in vitamin D concentrations with gestation (F(2, 58) = 7·26; P < 0·002) and an interaction with treatment (F(2, 58) = 5·37; P = 0·009) but no effect for treatment alone (F(1, 29) = 0·92; P = 0·347). Post hoc analysis showed that vitamin D concentrations were significantly lower in the placebo group compared with the treatment group at 26 weeks of gestation (P = 0·018). Although mean TDP concentration was higher for those receiving treatment at 34 weeks of gestation, this was non-significant.
At recruitment 13 % of women had anaemia (Hb < 110 g/l), 16 % low PCV concentrations ( < 0·330 litres per litre) and 11 % had low Fe stores (serum ferritin < 15 μg/l)(10). For all three measures of Fe status, the percentage of mothers with low status increased in later pregnancy; however, the increase was less amongst supplemented mothers. Of the women, seventeen (5 %) had marginal folate deficiency ( < 345 nmol/l) at booking, of which three had severe folate deficiency ( < 230 nmol/l)(Reference Sauberlich, Dowdy and Skala11). Folate status improved in later pregnancy, with only 1–2 % with low status. At recruitment 12 % of participants were thiamin deficient (TDP < 66·5 nmol/l); by 34 weeks of gestation this increased to 32 % in the placebo group compared with only 20 % in the treatment group.
Of participants at recruitment, 72 % had suboptimal 25-hydroxyvitamin D concentrations ( < 50 nmol/l)(Reference Malabanan, Veronikis and Holick12). Median 25-hydroxyvitamin D concentrations varied by ethnicity (P < 0·001; Kruskall–Wallis test); Caucasians had the highest and Asians the lowest (Table 6). Due to the small sample sizes at 26 and 34 weeks, Caucasians were compared with non-Caucasians; Caucasians had a higher median vitamin D concentration at 26 (P = 0·035; Mann–Whitney U test) but not 34 weeks of gestation. Median 25-hydroxyvitamin D levels were significantly greater in the summer (April to September) than the winter (October to March) (P = 0·006; Mann–Whitney U test) at 34 weeks of gestation, but not for the other occasions. At recruitment significant differences in median vitamin D concentrations were seen between the five ethnic groups for both summer (P < 0·001) and winter (P = 0·021; Kruskall–Wallis test). At 26 weeks Caucasians had a significantly higher median 25-hydroxyvitamin D concentration than non-Caucasians during the summer months (P = 0·018; Mann–Whitney U test) but not the winter; there were no differences by ethnicity and season at 34 weeks of gestation. Vitamin D status improved as pregnancy progressed, with low status being greatest amongst those receiving placebo. The small number of samples analysed for vitamin D at 26 and 34 weeks made it difficult to investigate the interaction between treatment, season, ethnicity and gestation on vitamin D status in further detail.
Table 6 Maternal vitamin D status by season* and ethnicity

* Summer is defined as April to September and winter is defined as October to March.
† Percentage by ethnic group.
Nutrient status and birth outcome
Gestational age at birth was weakly positively correlated with TDP concentrations at booking (P = 0·037; r 0·152) and at 34 weeks of gestation (P = 0·006; r 0·342). Head circumference was weakly positively associated with erythrocyte folate at recruitment (P = 0·046; r 0·111) and vitamin D concentrations at 34 weeks of gestation (P = 0·024; r 0·231). Serum ferritin at 26 weeks of gestation was weakly inversely correlated with head circumference (P = 0·021; r − 0·237). Infant birth weight was weakly inversely associated with PCV concentrations at 26 weeks of gestation (P = 0·024; r − 0·126).
Discussion
The present study demonstrated that multiple-micronutrient supplementation from the first trimester improved micronutrient status in later pregnancy, in this low-income, multi-ethnic population. Markers of Fe, folate, thiamin and vitamin D status were all higher during the third trimester for those receiving the supplement relative to the placebo group. Poor nutrient status during pregnancy and adverse obstetric outcome have been consistently seen throughout pregnancy in this population(Reference Doyle and Rees3).
Although the non-compliance rate was high, it is comparable with that seen previously in this population and reflects a lack of interest in health and poor knowledge of the relationship between nutrition and pregnancy outcome(Reference Doyle, Crawford and Srivastava13). One potential issue of the supplements is the formulation; thirty-one participants (8 % of all participants) ceased the study as they disliked the formulation of the supplements, the majority finding them too large to swallow. However, despite the low compliance, the treatment showed significant improvement in nutrient status relative to the placebo.
Considering only the participants who were compliant throughout the study, the supplement appeared to reduce the numbers of SGA infants. However, this was not seen using intention-to-treat analysis or only for women recruited before 13 weeks of gestation and will need further verification with much larger studies. Also there were weak associations between nutrient status and birth outcome. However, the present study was powered to investigate the effect of multiple-micronutrient supplementation on birth weight; thus a limitation of the results presented here is that the study was not powered to investigate effects on micronutrient status or other birth outcomes.
The prevalence of low Fe status at recruitment in the present study was similar to a previous Hackney study (9 % anaemic and 10 % low Fe stores)(Reference Rees, Brooke and Doyle4). Hb and PCV concentrations are known to decrease from the first to the third trimester and increase slightly at term(Reference Scholl14); this trend was clearly seen in the present study, however, the decrease was greatest for women receiving the placebo. Serum ferritin usually decreases as pregnancy advances(Reference Kaufer and Casaneuva15). In the present study as pregnancy progressed the number of participants with low Fe stores increased; however, this increase was less for supplemented women.
Research suggests that even women with good Fe status may benefit from low-dose Fe supplementation during pregnancy. Daily supplementation of Fe-replete women with 30 mg Fe from mid-pregnancy reduced the incidence of LBW and preterm birth(Reference Cogswell, Parvanta and Ickes16). The current supplement provided 20 mg Fe per d, higher than the UK reference nutrient intake (RNI) of 14·8 mg/d(17) but much lower than commonly prescribed during pregnancy (up to 300 mg/d). Patients often show poor compliance with high-dose Fe supplements, thought to be a result of gastric side effects such as heartburn, nausea, vomiting, constipation and diarrhoea(Reference Beard18). Similar numbers of women from both groups (twenty in the treatment group v. twenty-two in the placebo group) ceased the present study due to reported gastric side effects; these were most likely symptoms of pregnancy rather than actual side effects. Similarly Makrides et al. (Reference Makrides, Crowther and Gibson19) found that women supplemented with 20 mg Fe daily from 20 weeks of gestation until delivery had improved Fe status with no difference in gastrointestinal side effects compared with the placebo group. Further Zhou et al. (Reference Zhou, Gibson and Crowther20) demonstrated a daily dose of 20 mg elemental Fe during pregnancy resulted in reduced occurrence of nausea, stomach pain and vomiting compared with 40 or 80 mg Fe with no difference in the incidence of anaemia.
The prevalence of folate deficiency at recruitment within the study population has been discussed previously(Reference Brough, Rees and Crawford9) and is similar to the prevalence of deficiency seen in non-pregnant women in the UK National Diet and Nutrition Survey (NDNS)(Reference Ruston, Hoare and Henderson21). The supplement provided the recommended 400 μg folic acid per d(22) and as pregnancy progressed mean erythrocyte folate concentration increased amongst those using the supplement; however, those receiving the placebo showed a decrease in folate status as pregnancy advanced.
In the present study 12 % of participants were thiamin deficient during the first trimester, much higher than the 1 % of women seen in the NDNS(Reference Ruston, Hoare and Henderson21). The present treatment provided 3 mg thiamin/d, which is higher than the RNI(17) of 0·8–0·9 mg/d, but is not excessive compared with the US RDA(23) of 1·4 mg/d with no toxic level of intake defined. By 34 weeks of gestation participants using the supplement showed a non-significant increase in thiamin status. The proportion of women who were thiamin deficient increased in later pregnancy; however, this increase was less for the treatment group.
There is much debate surrounding the appropriate cut-off for optimal vitamin D status. Deficiency is typically defined as 25-hydroxyvitamin D < 25 nmol/l(24); however, Malabanan et al. (Reference Malabanan, Veronikis and Holick12) advocate < 50 nmol/l as insufficiency. Using these cut-offs in the present study levels of deficiency were 27, 22 and 14 % and levels of insufficiency were 70, 52 and 36 % at recruitment, 26 and 34 weeks of gestation, respectively, with a median concentration of 37·5, 47·0 and 62·0 nmol/l at each time-point. Using the same cut-offs, Holmes et al. (Reference Holmes, Barnes and Alexander25) found lower status among pregnant women in Northern Ireland, with levels of deficiency of 35, 44 and 16 % and levels of insufficiency of 96, 96 and 75 % at 12, 20 and 35 weeks of gestation. The present study shows considerable insufficiency amongst this multi-ethnic population which could result in adverse Ca handling for both mother and fetus. The major source of vitamin D is from sun exposure; however, highly pigmented skin is less effective at producing vitamin D(Reference Matsuoka, Wortsman and Haddad26). Also some ethnic groups restrict exposure to sunlight by covering up with clothing for cultural or religious reasons. Further, in the UK there is no appropriate UVB radiation between the end of October and the end of March(17). In the present study blood samples were collected throughout the year, which should minimise the effect of season on differences between treatment and placebo, although any effect cannot be ruled out.
The supplement provided 5 μg cholecalciferol (vitamin D3) per d, half of the RNI during pregnancy(24); this appears to have improved vitamin D status. Hollis & Wagner(Reference Hollis and Wagner27) argue that the current recommendations are insufficient and the appropriate dose could be as high as 250 μg/d. Hollis & Wagner(Reference Hollis and Wagner28) have demonstrated that high doses of vitamin D (100 or 50 μg/d) given to lactating women improve vitamin D status without adverse effects. Mannion et al. (Reference Mannion, Gray-Donald and Koski29) demonstrated in Canada at 51°N (the same latitude as Southern England) that dietary intake of vitamin D during pregnancy was a significant predictor of infant birth weight. The Royal College of Obstetricians and Gynaecologists and the National Institute for Health and Clinical Excellence suggest that it may be advisable for pregnant women to be supplemented daily with 10 μg vitamin D(30, 31); however, there is still a lack of awareness of the need for vitamin D during pregnancy(Reference Sharma, Khan and Khadri32). In the present study the only significant effect of treatment was at 26 weeks; the lack of a consistent effect could result from the supplement providing insufficient vitamin D for a population of such low status. It was not possible to establish the effects of ethnicity and season due to the small number of samples. Further work is required investigating vitamin D requirements during pregnancy, especially for different ethnic groups, in the light of recent studies suggesting the inadequacy of current recommendations and the relationship between status and birth outcome.
Conclusions
The present study showed that prophylactic use of a multiple-micronutrient supplement from the first trimester improved nutrient status, for measures of Fe, folate, vitamin D and thiamin, amongst a socially deprived, multi-ethnic population. It also adds weight to the argument that treatment with prophylactic low-dose Fe supplements may be preferable to using high doses for improving Fe status during pregnancy. The present study further suggests that such supplementation may improve fetal growth but further larger studies are required to corroborate these results, especially amongst disadvantaged populations within developed countries.
Acknowledgements
The study was supported with funding provided by the Mother and Child Foundation (http://www.motherandchildfoundation.org/). The multiple-micronutrient supplement and placebo tablets were manufactured and provided by Vitabiotics (London, UK). Funding for vitamin D analysis was provided by Nutricia Research Foundation (http://www.nutricia-research-foundation.org/).
The present study was carried out jointly by the Institute of Brain Chemistry and Human Nutrition (London Metropolitan University, London, UK) and Homerton University Hospital (London, UK).
L. B., G. A. R., M. A. C. and E. K. D. designed the study. L. B. and G. A. R. recruited participants and collected data. L. B. analysed the data and R. H. M. provided statistical advice. All authors contributed to the writing and reviewing of the paper and approved the final version.
L. B. and G. A. R. were formerly affiliated to London Metropolitan University.
There are no conflicts of interest.











