Hepatic steatosis results mainly from increased delivery of NEFA to the liver, and from a misbalance in endogenous lipid synthesis and catabolism in hepatocytes. Hepatic steatosis can lead to fatty liver disease. Non-alcoholic fatty liver disease is characterised by fatty infiltration of the liver, inflammation, hepatocellular damage and fibrosis. Non-alcoholic fatty liver disease is considered as the hepatic manifestations of the metabolic syndrome. The metabolic syndrome refers to a cluster of features including obesity, glucose intolerance, dyslipidaemia and hypertension(Reference Varela-Rey, Embade and Ariz1, Reference Marra, Gastaldelli and Baroni2).
Carbon tetrachloride (CCl4) is a highly toxic chemical agent which is used as an industrial solvent. CCl4 is widely used to induce hepatic steatosis and to study the effects of protective agents, especially antioxidants. The toxic effects of CCl4 on the liver have been extensively studied(Reference Cotran, Kumar, Collins, Cotran, Kumar and Collins3–Reference Masaki, Yamada and Ogata8). Metabolic activation of CCl4 by cytochrome P450 to the free radicals, namely trichloromethyl peroxy radicals, is reported to enhance lipid peroxidation and protein oxidation in the liver, resulting in widespread membrane damage and liver injury. Membrane damage also causes alterations in lipoptotein secretion and accumulation of lipoprotein and lipid droplets in hepatocytes(Reference Cotran, Kumar, Collins, Cotran, Kumar and Collins3–Reference Junnila, Barak and Rahko5).
Epidemiological observations have shown that a high intake of fruits and vegetables has health benefits against some types of cancer and cardiovascular disorders(Reference Ness and Powless9–Reference Parlakpinar, Olmez and Acet11). Their health-promoting properties are due to high vitamin, phytochemical (carotenoids, flavonoids, etc) and fibre content. Vitamins and phytochemicals show antioxidant, anti-inflammatory and anti-atherogenic effects in vivo (Reference Krinsky and Johnson10, Reference Gil, Tomas-Barberan and Hess-Pierce12–Reference Tipoe, Leung and Hung14).
The dietary intake of vitamins, carotenoids and flavonoids, which are widely distributed in fruits, could be beneficial in protecting against hepatotoxicity(Reference Williams15, Reference Galati, Mondello and Lauriano16). Apricot (Prunus armeniaca L.) is a fruit which has a high content of carotenoids, mainly β-carotene. β-Carotene is the source of provitamin A. A quantity of 250 g fresh or 30 g dried apricots provides 100 % of the recommended daily allowance of provitamin A(Reference Ruiz, Egea and Tomas-Barberan17). Apricot also contains vitamins C and E and Se(Reference Munzuroglu, Karatas and Geckil18). β-Carotene and vitamins C and E are well-known dietary antioxidants(Reference Diplock, Charleux and Crozier-Willi19, Reference Benzie20). Vitamins show other beneficial effects in vivo besides their antioxidant properties. Vitamin A (retinol) is the precursor of retinal, the light-sensitive group in rhodopsin and other visual pigments. A deficiency of this vitamin leads to night blindness(Reference Berg, Tymoczko, Stryer, Berg, Tymoczko and Stryer21). Vitamin C has cofactor activity for several important enzymes(Reference Diplock, Charleux and Crozier-Willi19). Se is an essential dietary trace element and shows antioxidant properties(Reference Naziroglu, Karaoglu and Aksoya22, Reference Ozturk, Ozturk and Batcioglu23).
There are data about the beneficial effects of β-carotene and vitamins as antioxidants(Reference Krinsky and Johnson10, Reference Krinsky24), but their effects when consumed in foods are not well defined. Apricot is a stone fruit and it is widely produced in Malatya province, situated in the eastern region of Turkey. According to recent statistics, about 80 % of apricot production originates from this region and 95 % of this production is marketed as dried apricots(Reference Akinci and Olmez25). The region has about 8 million apricot trees, 17 % of which are of the ‘Kabaasi’ cultivar(Reference Asma26). Especially the ‘Hacihaliloglu’, ‘Cologlu’, ‘Zerdali’ and ‘Kabaasi’ cultivars have high antioxidant capacity, in vitro (Reference Guclu, Altun and Ozyurek27).
In the present study, our aim was to investigate the long-term effect of apricot feeding on hepatic steatosis and damage induced by CCl4.
Experimental methods
Reduced glutathione (GSH), β-NADPH, sodium azide, 5′5-dithiobis(2-nitrobenzoic acid), nitroblue tetrazolium, 2-thiobarbituric acid and cytochrome c were obtained from Merck (Darmstad, Germany). All other chemicals were obtained from Sigma Chemical Co. (St Louis, MO, USA) unless otherwise stated.
Preparation of 10 % and 20 % apricot-containing rat pellet feed
The naturally sun-dried ‘Kabaasi’ cultivar (produce of Malatya, Turkey, obtained from Karapinar Kirlangic Village Agricultural Cooperative) was used in the present study because of its high radical-scavenging power (RSP). The rat feeds containing 10 % and 20 % natural dried apricots were prepared in Elazig Feed Factory (Elazig Yem Sanayii, Elazig, Turkey). The metabolic energy value was regulated to 11 095 kJ/kg (2650 kcal/kg) in the 10 % and 20 % apricot-containing feeds like the standard rat pellets. The composition of the feeds is presented in Table 1.
Table 1 The composition of the standard pellet and the 10 % and 20 % apricot-containing feeds (g/kg)
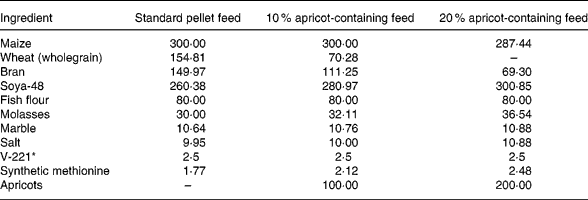
* V-221 is a mixture of vitamins and minerals.
Extract preparation
Standard pellet feed, apricot-containing feeds and sun-dried apricot samples (10 g) were mixed with 90 ml ethanol (70 %) and were crushed by using an Ultra-Turrax homogeniser (Ultra-Turrax, model T25; IKA-Works, Inc., Wilmington, NC, USA) at 12 000 g. The suspensions were kept in the refrigerator (+4°C) for 48 h and filtered through Whatman no. 1 filter paper. Extracts were used in the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging test.
1,1-Diphenyl-2-picrylhydrazyl radical-scavenging power assay
The RSP of standard pellet feed, apricot-containing feeds and dried apricot extracts were assessed by the method of Shimada et al. (Reference Shimada, Fujikawa and Yahara28) with slight modifications in 2 ml of reaction mixture containing 2·9 mmol DPPH (1·8 ml 1 × 10− 4 m-DPPH) and 0·2 ml of extracts. In the control, ethanol (70 %) was used in place of the sample. Cuvettes were left in the dark at room temperature for 10 min and the resulting colour was measured spectrophotometrically at 520 nm against blanks. A decreasing intensity of purple colour was related to higher RSP percentage, which was calculated using the following equation:
where A S:10 is the absorbance of the samples and A B:10 is the absorbance of the blank at the 10th min of the reaction period.
Animals and experimental groups
Adult male Wistar rats (n 42) weighing 250–350 g were provided by the Inonu University Laboratory Animals Research Centre. Animals were fed with a standard rat feed and tap water ad libitum for 10 d and randomly divided into six groups of seven each, as follows: control group (308·0 (sd 22·7) g); CCl4 group (310·6 (sd 32·2) g); CCl4+10 % apricot group (314·0 (sd 25·8) g); CCl4+20 % apricot group (313·0 (sd 37·5) g); 10 % apricot group (312·1 (sd 34·9) g); 20 % apricot group (309·4 (sd 22·8) g). The experiment lasted for 5 months.
The control and CCl4 groups were fed by the standard rat pellet, the 10 % apricot group and the CCl4+10 % apricot groups were fed by 10 % apricot-containing pellets, and the 20 % apricot group and the CCl4+20 % apricot groups were fed by 20 % apricot-containing pellets during 5 months. The CCl4, CCl4+10 % apricot and CCl4+20 % apricot groups received CCl4 at 1 ml/kg (dissolved in olive oil) (Raftel Kimya, Istanbul, Turkey) subcutanously for 3 d at the end of the 5 months. At 2 d after the last injection all rats were killed and livers were quickly removed.
All experiments in the present study were performed in accordance with the guidelines for animal research from the National Institute of Health and approved by the Committee on Animal Research at Inonu University, Malatya (2006/003).
Determination of liver function tests
Alanine amino transferase (ALT) and aspartate amino transferase (AST) activities were measured in serum samples obtained from all groups of rats. The measurements were done in accordance with the methods of the diagnostic kits (Biolabo Reagents, Maizy, France).
Lipid peroxidation assay (malondialdehyde)
The analysis of lipid peroxidation was carried out as described(Reference Buege and Aust29) with a minor modification. The reaction mixture was prepared by adding 250 μl homogenate into 2 ml reaction solution (15 % trichloroacetic acid–0·375 % thiobarbituric acid–0·25 m-HCl, 1:1:1, w/w/v) and heated at 100°C for 15 min. The mixture was cooled to room temperature, centrifuged (10 000 g for 10 min) and the absorbance of the supernatant fraction was recorded at 532 nm. 1,1,3,3-Tetramethoxypropane was used as a malondialdehyde (MDA) standard. MDA results were expressed as nmol/mg protein in the homogenate.
The protein content of samples was determined using the colorimetric method of Lowry et al. (Reference Lowry, Rosenbrough and Farr30) using BSA as the standard. All analyses were performed in duplicate.
Determination of enzyme activities
Catalase assay
Catalase (CAT) activity was measured at 37°C by following the rate of disappearance of H2O2 at 240 nm (ɛ240 = 40/m per cm)(Reference Luck and Bergmeyer31). The definition of 1 unit of CAT activity is the amount of enzyme catalysing the degradation of 1 μmol H2O2/min at 37°C and specific activity corresponding to transformation of substrate (in μmol) (H2O2)/min per mg protein.
Superoxide dismutase assay
Superoxide dismutase (SOD) activity was assayed using the nitroblue tetrazolium method of Sun et al. (Reference Sun, Oberley and Li32). The samples were subjected to ethanol–chloroform extraction before the enzyme activity assay. Briefly, 250 μl ice-cold ethanol–chloroform mixture (62·5:37·5, v/v) was mixed thoroughly with 250 μl of sample. After vortexing for 30 s and centrifuging at 10 000 g at 4°C for 30 min, the upper aqueous layer was collected. The collected supernatant fraction was diluted by a factor of 10, and 50 μl of the diluted solution was used for the assay by adding it to 2·9 ml of SOD assay reagent and 50 μl of xanthine oxidase solution (0·16 U/ml). Nitroblue tetrazolium was reduced to blue formazan by O2 − , which has a strong absorbance at 560 nm. The definition of 1 unit of SOD is the amount of protein that inhibits the rate of nitroblue tetrazolium reduction by 50 %. The calculated SOD activity was expressed as U/mg protein in the tissue.
Glutathione peroxidase assay
Glutathione peroxidase (GSH-Px) activity was determined in a coupled assay with glutathione reductase by measuring the rate of NADPH oxidation at 340 nm using H2O2 as the substrate(Reference Lawrence and Burk33). The enzyme reaction contained 30 mm-potassium phosphate, pH 7·0, 1 mm-EDTA, 0·2 mm-NADPH, 2 mm-GSH, 1 mm-sodium azide, 1 U glutathione reductase and 0·1 mm-H2O2. The definition of 1 unit of GSH-Px is the amount of NADPH (μmol) disappeared/min per mg protein.
Total glutathione assay
The total glutathione (TGSH) level was performed using the method of Theodorus et al. with some modifications(Reference Theodorus, Akerboom and Sies34). The reaction mixture contained 50 mm-sodium phosphate, 1 mm-EDTA, 0·5 mm-5′5-dithiobis(2-nitrobenzoic acid), 0·2 mm-NADPH and glutathione reductase (0·5 U/ml). Homogenate (10 μl) was added to initiate the reaction but was omitted for the control. The formation of 5-thio-2-nitrobenzoate was followed spectrophotometrically at 412 nm. The amount of TGSH in the extract was determined as nmol/mg protein utilising a commercial GSH as the standard.
Histological examination
For light microscopic evaluation, liver samples were fixed in phosphate-buffered 10 % formalin. Paraffin-embedded specimens were cut into 5 μm thick sections and stained with Crossmon's triple staining(Reference Crossmon35, Reference Presnell and Schreibman36). The livers were examined with a Leica DFC 280 light microscope by an experienced observer unaware of the animal treatment groups. The Leica Q Win Plus Image Analysis System (Leica Micros Imaging Solutions Ltd, Cambridge, UK) was used for morphometric analysis. Centrilobular necrosis areas (the areas full of vacuolated and necrotic hepatocytes) were measured in ten different fields for each section.
For transmission electron microscopic evaluation the samples were placed into 3 % glutaraldehyde buffered with 0·2 m-NAH2PO4+NaHPO4 (pH 7·2–7·3) and post-fixed with 2 % osmium tetroxide (OsO4) and embedded in Araldite CY 212. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Zeiss Libra 120 (Carl Zeiss NTS GmBH, Oberkochen, Germany) transmission electron microscope.
Statistical analysis
Results were expressed as arithmetic mean values and standard deviations. Results were statistically analysed by the Kruskal–Wallis H test and were considered to be significant when P < 0·05. The differences between the groups were evaluated by the Mann–Whitney U test.
Results
1,1-Diphenyl-2-picrylhydrazyl radical-scavenging power assay
The DPPH RSP of the standard rat chow, the 10 % and 20 % apricot-containing rat chows and dried apricot are given in Table 2. The RSP of dried apricot and apricot-containing rat pellets were significantly higher than that of the standard rat pellets (P < 0·05).
Table 2 The 1,1-diphenyl-2-picrylhydrazyl (DPPH)-scavenging assay of standard rat pellets, 10 % and 20 % apricot-containing rat pellets and dried apricot
(Mean values and standard deviations)

* Mean value was significantly increased compared with that of the standard rat pellets (P < 0·05).
† Mean value was significantly increased compared with that of the 10 % apricot-containing rat pellets (P < 0·05).
‡ Mean value was significantly increased compared with that of the 20 % apricot-containing rat pellets (P < 0·05).
Liver function tests
The levels of ALT and AST in serum in all groups are presented in Table 3. ALT and AST levels were 10·3 and 52·5 IU/l in the control group, 173·4 and 302·4 IU/l in the CCl4 group, 88·2 and 159 IU/l in the CCl4+10 % apricot group and 55·8 and 177·7 IU/l in the CCl4+20 % apricot group, respectively. According to these results, we can say that apricot feeding protected liver from the effects of CCl4.
Table 3 The effects of carbon tetrachloride (CCl4) and apricot feeding on liver function tests after the feeding period
(Mean values and standard deviations)
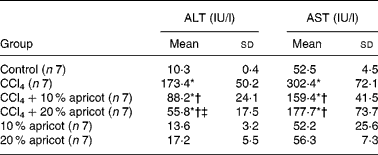
ALT, alanine amino transferase; AST, aspartate amino transferase.
* Mean value was significantly different that of the control group (P < 0·05).
† Mean value was significantly different that of the CCl4 group (P < 0·05).
‡ Mean value was significantly different that of the CCl4+10 % apricot group (P < 0·05).
Lipid peroxidation and enzyme results
The levels of MDA and TGSH and the activities of CAT, SOD and GSH-Px of the liver tissue are shown in Table 4. In the CCl4 group, MDA levels were increased (P < 0·05), and CAT, SOD and GSH-Px activities and TGSH levels were decreased significantly (P < 0·05) when compared with the control group. MDA levels were decreased in the CCl4+10 % and CCl4+20 % apricot groups when compared with the CCl4 group (P < 0·05). CAT, SOD and GSH-Px activities and TGSH levels were increased significantly in the CCl4+10 % and CCl4+20 % apricot groups when compared with the CCl4 group (P < 0·05). These results indicate that apricot feeding is effectively able to inhibit lipid peroxidation and the production of MDA, and inhibit the need and usage of antioxidant enzymes and/or stimulate the production of antioxidant enzymes in CCl4-injured liver.
Table 4 The effects of carbon tetrachloride (CCl4) and apricot feeding on biochemical parameters of liver tissue after the feeding period
(Mean values and standard deviations)
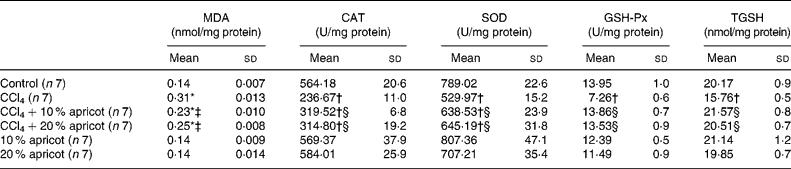
MDA, malondialdehyde; CAT, catalase; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; TGSH, total glutathione.
* Mean value was significantly increased compared with that of the control group (P < 0·05).
† Mean value was significantly decreased compared with that of the control group (P < 0·05).
‡ Mean value was significantly decreased compared with that of the CCl4 group (P < 0·05).
§ Mean value was significantly increased compared with that of the CCl4 group (P < 0·05).
Light microscopic observations
The livers of the control, 10 % apricot and 20 % apricot groups presented normal histology (Fig. 1(a)). The liver sections of the CCl4 group showed histological alterations such as vacuolated hepatocytes and hepatic necrosis, especially in the centrilobular area. (Fig. 1(b) and (c)). Inflammatory cell infiltration and slight fibrosis around the central vein were detected (Fig. 1(d)). In the CCl4+10 % apricot- and CCl4+20 % apricot-fed groups hepatic necrosis was rare. Vacuolated hepatocytes were seen in the centrilobular area but they were not as extensive as in the CCl4 group and their vacuoli were small (Fig. 1(e)). Inflammatory cell infiltration and fibrosis were rare. In the CCl4+10 % apricot and CCl4+20 % apricot groups, hepatocytes showed extensive mitosis (Fig. 1(f)). The histological findings of these two groups were close to each other. The morphometric analysis of the liver damage is presented in Table 5. The damaged area measurements showed that apricot feeding significantly decreased (P < 0·05) centrilobular necrosis induced by CCl4.
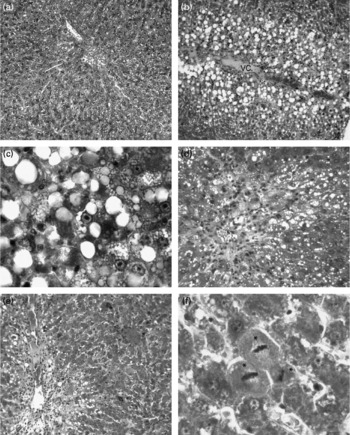
Fig. 1 Photomicrographs of rat livers (triple staining). In the control group (a, × 66), the liver has normal histology. In the CCl4 group (b–d) in the centrilobular area vacuolated hepatocytes (b, × 66), hepatocyte necrosis (c, × 330), slight fibrosis and inflammatory cell infiltration (d, × 132) are seen. In the CCl4+10 % apricot group (e, × 66) and the CCl4+20 % apricot group (f, × 330) vacuolisation is lessened, vacuoli are small and prominent mitotic hepatocytes are visible (*). VC, vena centralis.
Table 5 Morphometric analysis of liver damage in the control and experimental groups
(Mean values and standard deviations)
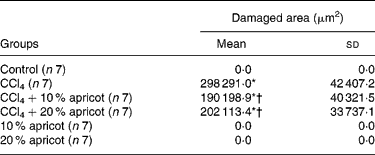
CCl4, carbon tetrachloride.
* Mean value was significantly increased compared with that of the control group (P < 0·05).
† Mean value was significantly decreased compared with that of the CCl4 group (P < 0·05).
Ultrastructural observations
The ultrastructure of hepatocytes was normal in the control, 10 % apricot and 20 % apricot groups (Fig. 2(a) and (b)). In the CCl4 group some of the hepatocytes showed large lipid globules and glycogen loss (Fig. 2(c)). Some hepatocytes showed an oedematous cytoplasmic matrix with large lipid globules, degenerated organelles and heterogeneous lysosomes. Mitochondrial changes such as swelling and cristae loss were seen (Fig. 2(d) and (e)). In both the CCl4+10 % apricot and the CCl4+20 % apricot groups, apricot feeding reduced the volume and number of the lipid globules in the hepatocytes. The organelle and cytoplasm structures were widely preserved from the effects of CCl4 (Fig. 2(f) and (g)). However, glycogen loss persisted in apricot-fed groups.
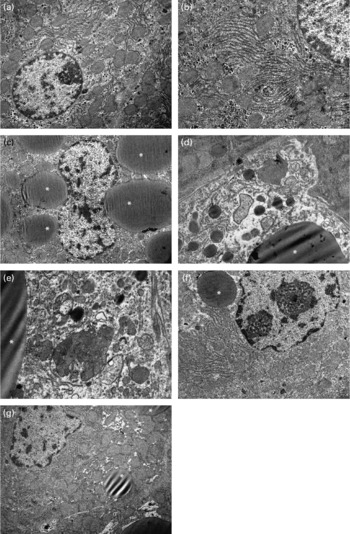
Fig. 2 Electron photomicrographs of rat livers. In the control group (a, × 8000) and the 10 % apricot group (b, × 12 500), hepatocytes show normal ultrastructure. In the CCl4 group, glycogen loss, large lipid globules (*) (c, × 6300), oedematous cytoplasmic matrix, degenerated organelles, swelled mitochondria with cristae loss and heterogeneous lysosomes (d, × 12 500; e, × 16 000) are seen. In the CCl4+10 % apricot group (f, × 8000) and the CCl4+20 % apricot group (g, × 8000), glycogen loss persists in hepatocytes, but lipid globules are small and few.
Discussion
Hepatic steatosis increases the sensitivity of the liver to secondary insults and leads to lipotoxicity. Lipotoxicity causes mitochondrial dysfunction, apoptosis, necrosis, generation of oxidative stress and inflammation(Reference Varela-Rey, Embade and Ariz1, Reference Marra, Gastaldelli and Baroni2). Mitochondrial impairment causes enhanced reactive nitrogen species production, initiating lipid peroxidation(Reference Varela-Rey, Embade and Ariz1).
Lipid peroxidation and oxidative damage are well defined in CCl4 hepatotoxicity. Cytochrome P450 activates CCl4 into the trichloromethyl radical, which further converts to the peroxy radical. These locally produced free radicals cause auto-oxidation of the polyenic fatty acids present within the membrane phospholipids. As a result of oxidative lipid decomposition and organic peroxide formation, lipid peroxidation occurs. Lipid export from the hepatocytes is reduced owing to their inability to synthesise apoprotein which facilitates lipoprotein secretion. This leads to fatty liver(Reference Cotran, Kumar, Collins, Cotran, Kumar and Collins3–Reference Junnila, Barak and Rahko5). Our findings in the CCl4 group are concordant with these data. We detected hepatocellular degeneration with vacuolisation and necrosis of hepatocytes which was severe in the centrilobular area. The reason of the hepatocyte vacuolisation was the lipid accumulation which was seen ultrastructurally as lipid globules in hepatocytes. Several studies have reported hepatic centrilobular steatosis and necrosis after exposure to CCl4(Reference Junnila, Barak and Rahko5, Reference Wang, Ma and Liu6, Reference Masaki, Yamada and Ogata8, Reference Sato, Dai and Liu37–Reference Noyan, Cavusoglu and Minbay39). The changes in organelle structure and oedematous cytoplasmic matrix in this group were probably due to the changes in membrane structure caused by lipid peroxidation. We also detected glycogen loss in hepatocytes. It is believed that high energy demand for the possible repair processes could lead to glycogen loss in CCl4-given animals(Reference Junnila, Rahko and Sukura40). In the present study the MDA levels were significantly increased in the CCl4 group (P < 0·05). MDA is a product of lipid peroxidation and increased levels of MDA are an indicator of lipid peroxidation. The intrinsic scavenger enzymes (CAT, SOD and GSH-Px) and TGSH were significantly decreased (P < 0·05) in the CCl4 group. The decreased activities of the antioxidative enzymes probably occured as a result of free radical production and the excessive use of these enzymes. Wang et al. (Reference Wang, Ma and Liu6) found increased MDA levels and decreased CAT, SOD and GSH-Px activities in CCl4-treated rats. ALT and AST levels were significantly increased (P < 0·05) in the CCl4 group. The increase in blood plasma levels of ALT and AST show liver damage.
Some natural foods are considered to have antioxidant and anti-inflammatory properties because of their contents such as vitamins and phytochemicals(Reference Gil, Tomas-Barberan and Hess-Pierce12–Reference Tipoe, Leung and Hung14, Reference Krinsky24, Reference Fujii, Yokozawa and Kim41). Anti-inflammatory properties of green tea and pomegranate juice are reported(Reference Basu and Penugonda13, Reference Tipoe, Leung and Hung14). In vivo nutrient investigations are mostly focused on their antioxidant properties(Reference Gil, Tomas-Barberan and Hess-Pierce12–Reference Tipoe, Leung and Hung14, Reference Fujii, Yokozawa and Kim41). The vitamin and phytochemical content of fruits show their antioxidant actions with different mechanisms. Vitamin C, vitamin E and flavonoids scavenge and destroy reactive oxygen species with their sacrificial and chain-breaking properties. Carotenoids act as chemical traps and quench reactive oxygen species(Reference Benzie20). In the present study the in vitro RSP of the standard diet, dried apricot and apricot-containing feeds were analysed. The dried ‘Kabaasi’ apricot showed the highest RSP. Guclu et al. (Reference Guclu, Altun and Ozyurek27) reported that the ‘Kabaasi’ cultivar has a high antioxidant capacity in vitro. Apricot is a fruit which has a high β-carotene content(Reference Ruiz, Egea and Tomas-Barberan17). Carotenoids are naturally occuring pigments in plants that are involved in light-harvesting reactions and protection of plant organelles against singlet oxygen-induced damage. Martin et al. (Reference Martin, Failla and Smith42) reported that β-carotene protected HepG2 human liver cells against oxidant-mediated changes in plasma membrane integrity. Carotenoids have singlet oxygen and peroxy radical-quenching properties because of their long chain of conjugated double bonds. β-Carotene may have additive benefits due to its ability to be converted to vitamin A. β-Carotene is the major plant source of vitamin A(Reference Krinsky and Johnson10, Reference Thurnham43). However, high-dose β-carotene supplementation may show pro-oxidative properties. Under heavy oxidative stress β-carotene and other carotenoids are oxidatively degraded leading to the formation of high amounts of breakdown products with pro-oxidant properties(Reference Siems, Wiswedel and Salerno44). These products may exert harmful effects. There are studies about the possible adverse effects of β-carotene on the incidence of lung cancer in heavy smokers(Reference Diplock, Charleux and Crozier-Willi19, Reference Omenn, Goodman and Thornquist45).
Apricot also contains vitamins C and E and Se(Reference Munzuroglu, Karatas and Geckil18). Vitamin C is considered to be one of the most powerful, least toxic natural antioxidants. It is water soluble and has cofactor activity(Reference Diplock, Charleux and Crozier-Willi19). It is an essential cofactor in the hydroxylation of procollagen chains during collagen synthesis(Reference Berg, Tymoczko, Stryer, Berg, Tymoczko and Stryer21). Vitamin E and Se also have antioxidant properties(Reference Diplock, Charleux and Crozier-Willi19, Reference Naziroglu, Karaoglu and Aksoya22, Reference Ozturk, Ozturk and Batcioglu23). We fed rats with 10 % and 20 % apricot-containing feed for 5 months before CCl4 exposure. These feeds showed protective effects in CCl4-induced liver injury. Apricot feeding reduced centrilobular necrosis area and preserved hepatocyte ultrastructure. ALT, AST and MDA levels decreased, and antioxidant enzyme activities increased significantly (P < 0·05) when compared with the CCl4 group. These results indicated that apricot feeding prevented lipid peroxidation and decreased oxidative stress.
According to epidemiological studies the regular consumption of fruits and vegetables is strongly associated with the reduced risk of developing chronic diseases. Their health-promoting properties are due to high vitamin, phytochemical and fibre content. However, the isolated single phytochemical does not exert the same beneficial health effect. It seems that combinations of phytochemicals are likely to show beneficial effects by their overlapping and complementary mechanisms. Thousands of phytochemicals are present in whole foods(Reference Liu46, Reference de Kok, van Breda and Manson47). Apricot is a phytochemical- and vitamin-rich fruit. The synergistic effect of these compounds could probably have played an important role in apricot's hepatoprotective effect in the present study.
There are studies about the hepatoprotective effects of natural foods and vitamins in CCl4-induced liver damage(Reference Galati, Mondello and Lauriano16, Reference Rusu, Tamas and Puica38, Reference Noyan, Cavusoglu and Minbay39). We detected prominent mitosis in hepatocytes of the CCl4+apricot group livers. Sato et al. (Reference Sato, Dai and Liu37) reported similar finding in their study. They detected mitosis in hepatocytes and Kupffer cells 48 h after CCl4 treatment in rats concomitantly administered hepatocyte growth-promoting factor. Liver has a high regeneration capacity and different cytokines, complete mitogens and co-mitogens are involved in regeneration(Reference Court, Wemyss-Holden and Dennison48). We detected regenerative effect of apricot in CCl4-induced liver damage. However, it is not possible to discuss the reasons of the mitosis induction with apricot feeding with the present findings. This issue needs further invesitigation.
In the present study we used two different feeds of 10 % and 20 % apricot concentration in order to compare their effects. The RSP of the dried apricot and apricot-containing feeds were found to be significantly higher than that of the standard rat feed. However, the RSP of the 10 % and 20 % apricot-containing feeds did not show significant differences from each other. Some studies showed that the DPPH-scavenging activity of nutrients reaches a plateau at a constant concentration and beyond this concentration the scavenging effect became almost stable(Reference Singh, Chidambara Murthy and Jayaprakasha49, Reference Singh, Singh and Singh50). We believe that the 20 % apricot-containing feed's RSP is probably at the plateau level. Further in vitro studies with different concentrations of apricot-containing feeds are needed for accurate results. The histological and biochemical findings were also similar in both apricot-fed groups. Only ALT levels showed significant difference between the CCl4+10 % and the CCl4+20 % apricot groups. According to the lipid peroxidation assay (MDA), antioxidant enzymes and histopathological findings, 20 % apricot did not improve the effect of 10 % apricot any further. These in vivo findings are concordant with the in vitro RSP results. The improved ALT levels of the 20 % apricot-containing feed could be due to non-antioxidant effects of apricot.
We conclude that long-term apricot feeding shows beneficial effects on CCl4-induced liver steatosis and damage in rats because of its high radical-scavenging capacity. Its effect occurred probably due to its high phytochemical and vitamin content. It is possible to say that apricot as a natural food could have beneficial effects on non-alcoholic hepatic steatosis.
Acknowledgements
The present study was supported by Inonu University Scientific Research Projects Unit (2005/70) and Malatya Province Apricot Research and Improvement Foundation (2006).
We would like to give special thanks to Bengul Gunes for her technical assistance.
The contributions of the authors are: F. O., project leader, investigation and evaluation of the light and electron microscopic findings, writer of the manuscript; M. G., investigation of electron microscopic findings; B. A. and I. Y., investigation of RSP, lipid peroxidation and antioxidant enzymes; C. O., investigation of liver function tests; A. O., preparation and evaluation of apricot feed; A. C. and N. V., manipulation of rats.
The authors state that there are no conflicts of interest.











