INTRODUCTION
Cryptosporidiosis is an important cause of enteric disease in Canada. Since 2000 (when national reporting began), the national incidence rate (per 100 000) for cryptosporidiosis has ranged from a low of 1·85 to a high of 2·67 (with a spike in 2001 of 7·47) [1]. The infection in Canada is presumed to be acquired from several possible sources, including drinking water supplies, recreational waters (both natural and swimming pools), as well as contact with animals, and person-to-person [Reference Yoder and Beach2]. During the study period, the annual incidence of cryptosporidiosis in Waterloo Region was almost double both the provincial and national rates [1].
Surveillance for enteric diseases in Canada is based primarily on the laboratory confirmation of a pathogen from patient stool samples submitted upon request of the attending physician. This passive system relies upon a symptomatic person seeking medical attention (about 24% of those ill), the physician requesting subsequent laboratory testing for enteric pathogens (requested in about 26% of cases seen by physicians), the laboratory testing for the appropriate pathogen and the test correctly identifying the pathogen (positive results occur in about 12% of patient samples received) [Reference Majowicz3]. In Canada, not all stool samples are examined for ova and parasites – this analysis must be requested by the attending physician. Epidemiological and laboratory data for all enteric cases are collected and evaluated at the local public health unit. The amount and type of information on each case varies by jurisdiction. Because of the lack of enhancement and standardization of patient interview tools, comparisons of risk factor information between jurisdictions across cases and time are often not possible. Thus, there is little current, reliable information available on routes of exposure and attribution of sources of enteric infections in Canada.
In response to these gaps in knowledge about the burden of enteric disease and source attribution in Canada, the Canadian Integrated Enteric Disease Surveillance System (C-EnterNet) was launched in 2005 in a pilot community in Ontario, Canada. The sentinel surveillance system design was based on the United States Centers for Disease Control (US CDC) FoodNet model [4]. The first sentinel site is located in the regional municipality of Waterloo, Ontario. The programme was designed to capture disease incidence in the human population while subsequently monitoring exposure sources (retail food, on-farm manure and surface water) [5]. One of the most important aspects of the sentinel surveillance approach involved the development of an enhanced enteric disease questionnaire that was adopted by the local health unit for all laboratory-confirmed enteric case follow-ups. The epidemiological data collected with this tool form the basis of this analysis.
METHODS
Outbreaks of enteric disease are generally analysed using a case-control study design, to assess the nature and magnitude of the relationship between exposures (risk factors) and disease [Reference Dohoo, Martin and Stryhn6]. An adaptation of the classic case-control design was used in this study, using as controls other non-Cryptosporidium-infected cases that were captured by the C-EnterNet surveillance system [Reference McCarthy and Giesecke7]. Here, a secondary-based study design was used, using C-EnterNet surveillance data for the analyses. Both cases and controls came from the C-EnterNet surveillance registry, which is one step removed from the actual source population [Reference Dohoo, Martin and Stryhn6]. These data included persons who resided in Waterloo Region and reported the date of onset of cryptosporidiosis between April 2005 and December 2007, who were classified as cases. Persons who reported date of onset with at least one of nine other enteric diseases during the same time period were classified as controls. Analysis was restricted to endemic, sporadic cases of disease (for both the case and control groups).
This modified case-control study was conducted over 32 months (the first 3 years of the enhanced surveillance initiative in Waterloo Region). The C-EnterNet pilot sentinel site is located in the regional municipality of Waterloo public health unit jurisdiction, including three cities (Cambridge, Kitchener, Waterloo) and four townships (Wilmot, Woolwich, Wellesley, North Dumfries). The population of Waterloo Region is 500 000, roughly 70% urban and 30% rural.
Persons with laboratory-confirmed Cryptosporidium infections were classified as cases. Non-cryptosporidiosis laboratory-confirmed enteric disease cases that occurred during the study period were classified as controls. These included amoebiasis, campylobacteriosis, cyclosporiasis, giardiasis, listeriosis, salmonellosis, shigellosis, verotoxigenic E. coli infections, and yersiniosis.
Exposure data were collected for 7 days prior to onset of illness for cases and variable periods between 7 and 28 days (depending on the disease) prior to onset of illness for controls, via a standardized questionnaire administered over the telephone by a public health inspector at the local health unit.
A causal diagram was developed to identify direct and indirect causal pathways and possible confounding in the measured predictor variables and outcome. All statistical analyses were performed with Stata for Windows version 10 (Stata Corporation, USA). Data screening was performed in Microsoft Excel 2003 (Professional Edition, Microsoft Corporation, USA). Patient records with missing data (including nil and ‘don't know’ responses) were omitted only from analyses pertaining to the question under investigation.
All reported laboratory-confirmed enteric cases were initially categorized into three groups: endemic, outbreak and international travel-related (within the week prior to illness). Seven age groups were assigned: 0–5, 6–12, 13–17, 18–24, 25–39, 40–59, and ⩾60 years. The reported date of onset was used to define the reported month and season of onset [spring (March–May), summer (June–August), autumn (September–November), winter (December–February)].
Univariable comparisons of demographic and clinical data between cases and controls were performed using Pearson's χ2 test, or Fisher's exact test if cell values were <5, and Student's t test or Wilcoxon–Mann–Whitney test for continuous predictors. Multivariable unconditional logistic regression was then performed using manual stepwise backward elimination. Variables that were significant based on initial univariable analysis (using a liberal P value of 0·3) were included in the four subset models. Analytical power was insufficient to allow for the fitting of a full model with all potential predictors. Instead, smaller surrogate models were built to address three routes of exposure based on a priori hypotheses for Cryptosporidium transmission (recreational water, environmental, and person-to-person transmission). Lack of power also precluded consideration of potential interactions. Confounding was considered to be important when a change of ⩾30% or more in model coefficients was identified upon removal of a predictor [Reference Dohoo, Martin and Stryhn6]. Diagnostic plots of residuals and influence measures were used to identify unusual patterns or influential respondents. Odds ratios and 95% confidence intervals (CI) are presented for associations between Cryptosporidium infection and various risk factors. The Hosmer–Lemeshow χ2 test was used to measure model goodness-of-fit. Receiver-operating characteristic (ROC) curves were generated for each model to illustrate their predictive power. Influential observations were identified using the Delta Beta statistic and outliers were identified with deviance residuals. The sensitivity and specificity for various probability cut-offs were graphically assessed. Linearity for the one continuous variable (age) was assessed by comparing the continuous variable grouped into quartiles and with a graphical approach using the kernel-smoothing graph procedure.
RESULTS
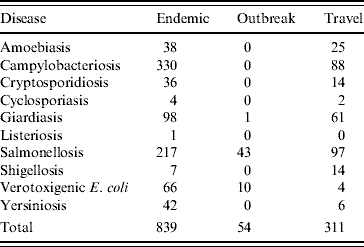
Epidemiological data were collected for 1204 cases of enteric illness in the sentinel area between April 2005 and December 2007, of which 839 were classified as endemic (domestically acquired, either within Waterloo Region or during travel in Canada), 54 were associated with outbreaks and 311 were travel-related (any travel outside Canada) (Table 1). Both outbreak and travel-related cases were excluded from the analysis. The final analysis included 36 endemic cases and 803 endemic controls.
Table 1. Classification of enteric disease cases for study period (2005–2007) in Waterloo Region
During the study period, the cryptosporidiosis incidence rate was 4·26/100 000 person-years in Waterloo Region. In comparison, between 2005 and 2006 in Canada and Ontario, the average incidence rates were 2·1/100 000 and 2·5/100 000, respectively [8]. The national and provincial rates for 2007 were unavailable at the time of this publication.
Case and control groups
Cases and control groups were similar regarding gender (P=0·36) and mean duration of illness (10 days vs. 10·1 days, P=0·96), but differed by mean age (cases 21·7 years, controls 31·8 years, P=0·01). When age of onset was categorized, cases were significantly more likely to be aged <6 years (25% vs. 18%) or aged 6–12 years (28% vs. 10%) than controls, and controls were significantly more likely to be aged >40 years (35% of controls vs. 17% of cases (Table 2). Cases and controls also differed by some disease symptoms: cases were more likely to have reported vomiting (P=0·001) and malaise (P=0·06), and less likely to have reported hospitalization (P=0·07) (Table 2). Cases and controls also differed by season of onset. Fewer cases were reported in the spring and winter (6%) than controls (26%) (Fig. 1), although this difference was not statistically significant at the 0·05 level (Table 3).
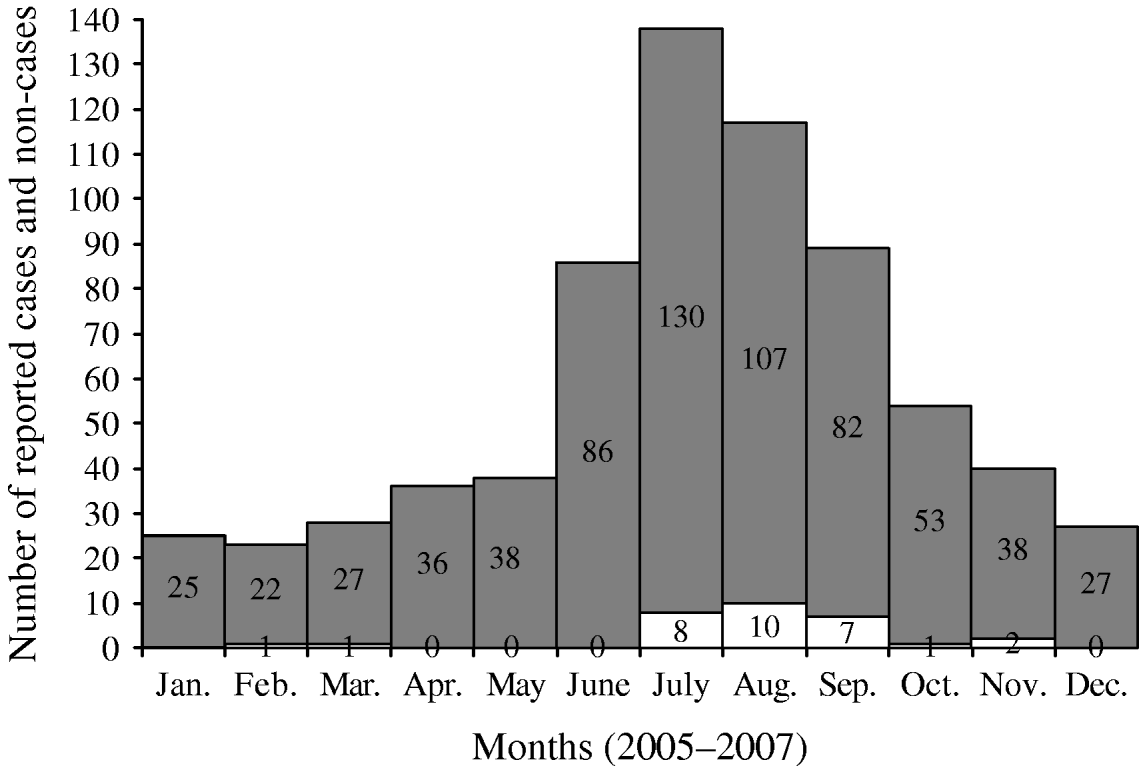
Fig. 1. Monthly distribution of cases (□) of cryptosporidiosis and controls (![]() ) during the study period.
) during the study period.
Table 2. Demographics and symptoms of endemic cryptosporidiosis and non-cryptosporidiosis enteric cases (April 2005–December 2007)
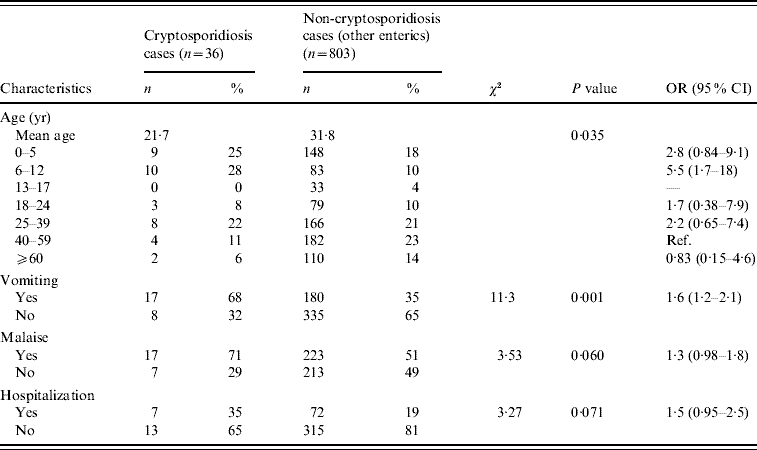
OR, Odds ratio; CI, confidence interval.
Table 3. Significant exposure factors within previous week for Cryptosporidium infection by univariate (single risk) analysis (April 2005–December 2007)
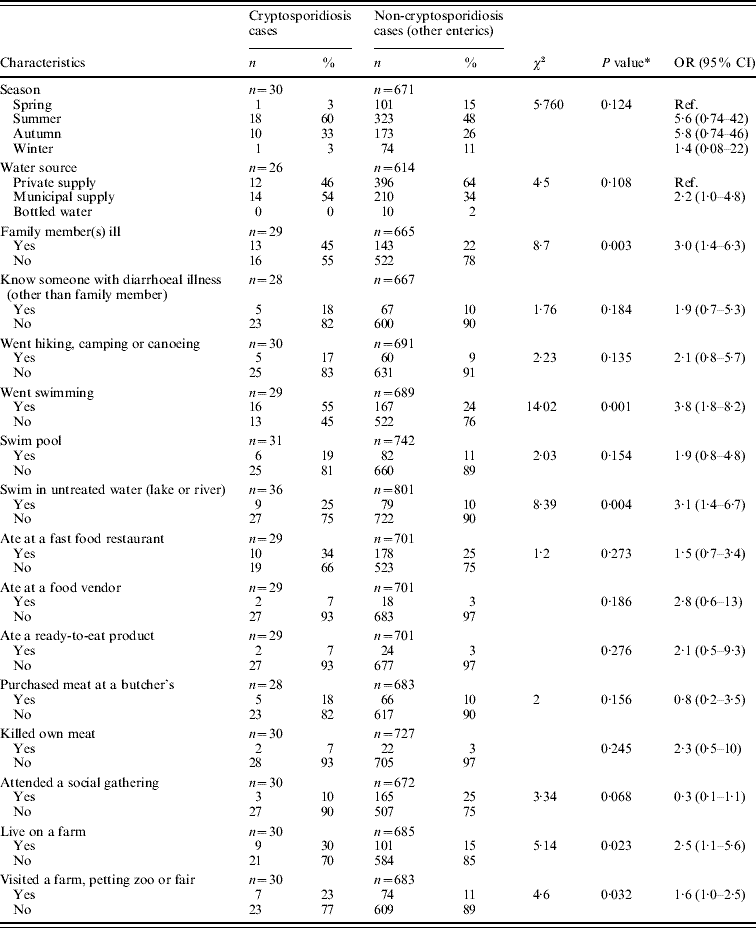
OR, Odds ratio; CI, confidence interval.
* Exposures where P<0·3 are presented.
None of cryptosporidiosis cases reported contact with an ill household pet, and only one case reported contact with animal faeces.
Univariable analyses
Statistically significant associations (P<0·05) were found between cryptosporidiosis and the following exposure factors: having a family member with a diarrhoeal illness, swimming (including swimming in a lake or river), living on a farm, and visiting a farm, petting zoo or fair (Table 3). Exposures that remained significant at a liberal P value of 0·3 are listed in Table 3 and were used to inform further multivariable analyses.
Multivariable analyses
Regression results of the four multivariable models that were developed are presented in Table 4.
Table 4. Logistic model results for four exposure pathways
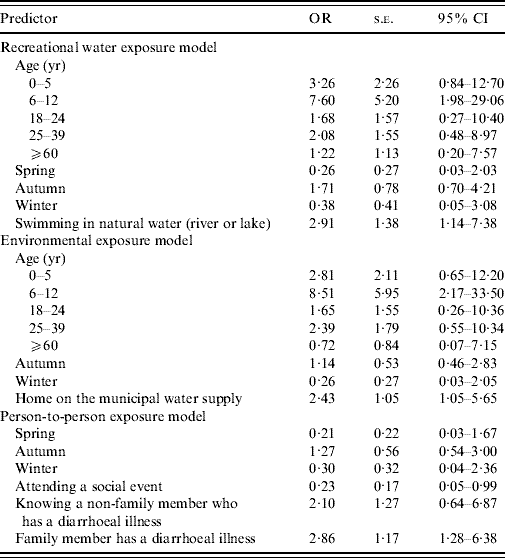
OR, Odds ratio; CI, confidence interval.
Recreational water exposure
Based on the initial causal diagram, it was hypothesized that age and season would confound the association between factors of interest and disease. In the final model, age and season were significant, as tested by the likelihood ratio test. Adjusting for age and season, swimming in an untreated water venue (river or lake) (P=0·01) was associated with a threefold increase in the odds of acquiring cryptosporidiosis (95% CI 1·2–7·4). The model fit the data, according to the Hosmer–Lemeshow χ2 test (P=0·49) and the predictive ability of the test was moderate (area under the curve=73·9%).
Environmental exposure
Adjusting for age and season, primary drinking water source (either private well or municipal water) was significant in the final model. Use of the municipal water source was associated with a 2·4-fold increase in the odds of acquiring cryptosporidiosis (95% CI 1·04–5·7) (P=0·04), compared to use of a private water source (bottled water source was omitted in the final model since it predicted failure perfectly). Other sources of exposure, through contact with animals, such as living on or visiting a farm, petting zoo or fair, were investigated but were not found to be significantly associated with the outcome. The model fit the data, according to the Hosmer–Lemeshow χ2 test (P=0·43), and the predictive ability of the test was moderate (area under the curve=76%).
Person-to-person exposure
Season was found to affect the model, as tested by the likelihood ratio test, although not significant in the final model by the Wald test. Adjusting for season, having a family member with a diarrhoeal illness (P=0·01) was associated with a 2·9-fold increase in the odds of acquiring cryptosporidiosis (95% CI 1·3–6·4). Knowing someone with a diarrhoeal disease (that was not a family member) was not significant at the 0·05 level by the Wald test but did affect the model, as tested by the likelihood ratio test, and was retained. Knowing a non-family member with a diarrhoeal disease was associated with a 2·1-fold increase in the odds of acquiring the disease, although the confidence interval bridged 1 (95% CI −0·6 to 6·9). Attending a social event during the exposure period was associated with a 0·23-fold (95% CI 0·05–0·99) decrease in the odds of acquiring the disease. The model fit the data, according to the Hosmer–Lemeshow χ2 test (P=0·77), and the predictive ability of the test was moderate (area under the curve=73%).
DISCUSSION
This analysis was performed to develop plausible hypotheses to explain the observed cryptosporidiosis in the target population, using an alternative control population in a case-control study. We assumed no secular trend over the 3-year study period. No major public health interventions or policy decisions were implemented in the study area during that time period that might have affected the incidence of cryptosporidiosis.
The results of this study illustrate that, in Waterloo Region, swimming in a river or lake, consuming municipal water (a potential surrogate for urban respondents vs. rural), and having a family member with a diarrhoeal illness are associated with an increased risk of developing cryptosporidiosis. Residents aged <12 years were more likely to acquire the disease, and cases were more common in autumn.
Increased risk in younger cases (aged <12 years) in this study can be attributed to contact with infected people and person-to-person transmission in household or daycare settings, possibly associated with diaper use and poor hand hygiene. Cryptosporidiosis is considered to be more common in children aged <5 years [Reference Majowicz9–Reference Dietz and Roberts11] and the US CDC surveillance data follow a bimodal age distribution with increased incidence in children aged <9 years and adults aged 30–39 years [Reference Yoder and Beach2]. The UK has reported a decline in the percentage of cryptosporidiosis cases observed in those aged <5 years and an increase in the percentage in older age groups [Reference Nichols12], but it is still unclear whether this is due to increased testing of older individuals or reduced exposure in the <5 years age group.
In this study, cases were twice as likely to acquire the disease in autumn. Cryptosporidiosis is seasonal in North America, with higher numbers in late summer and early autumn [Reference Yoder and Beach2]. UK data suggest that this seasonality is genotype-specific, and that cases of C. parvum are more prevalent in spring to early summer while C. hominis is more prevalent in late summer and autumn [Reference Nichols12]. Currently in Canada, routine molecular subtyping of Cryptosporidium-positive stool samples is not performed and comparisons can not be made with other countries.
Environmental exposure through recreational water contact emerged as a significant risk factor for cryptosporidiosis. Cryptosporidium is an ideal waterborne pathogen since it is (a) environmentally resilient, (b) has a low infectious dose, and (c) is resistant to desiccation and chlorine [Reference Mathieu13]. In this analysis, swimming in a lake or river was significantly associated with an increase in the odds of Cryptosporidium infection.
Understanding the role swimming (particularly in natural water venues) plays in Cryptosporidium infection could inform future public education initiatives around limiting the use of recreational venues when a person has diarrhoea (and for 2 weeks post-diarrhoea), as per the new US CDC regulations [14]. In addition, reducing the consumption of water while swimming, particularly in lakes and rivers, would reduce risk. Full body immersion is associated with an increased risk of acquiring the disease, in comparison with wading [15].
In natural water venues, there are a number of sources for human-infective strains of Cryptosporidium in the environment that could contribute to contamination and an increased risk of disease, including agricultural run-off, wastewater treatment effluent, and wildlife [Reference Perz and LeBlancq16]. Interventions to reduce the level of Cryptosporidium oocysts in rivers and lakes must be done at the watershed level, including additional treatment of human sewage, appropriate management of animal facilities and manure storage sites, the prevention of livestock access to watercourses, and the adoption of source-water protection plans [17].
While swimming in a pool did not emerge as a significant risk, it is considered to be one of the most important causes of both sporadic cases and outbreaks of cryptosporidiosis in North America [1, Reference Semenza and Nichols10, Reference Mathieu13, Reference Hunt18–Reference McAnulty, Fleming and Gonzalez20]. Cryptosporidiosis outbreaks have also been associated with recreational lake exposure in New Jersey and Illinois [Reference Kramer21, Reference Causer22]. In the UK, outbreaks have been linked to recreational use of a river, stream and beach [Reference Nichols12]. In our analysis, the limited power and use of ill cases as controls may have affected the ability to identify pools as a risk factor. In addition, the sporadic nature of the cases might have an influence on the role of treated vs. untreated swimming venues. There were no outbreak-related Cryptosporidium cases identified during the study period. These results do not support an association of pool swimming with the acquisition of cryptosporidiosis in Waterloo Region. However, it would be prudent to continue to consider pools as an important potential source of infection, based on surveillance and outbreak reports from other jurisdictions in North America. Pools have been shown to be important secondary transmission routes for community infections.
Cryptosporidium acquired via food, although possible, is not as commonly reported as waterborne infections. In the most recent US CDC surveillance summary of foodborne disease (between 1998 and 2002), only 0·1% of cryptosporidiosis cases in the country were attributed to food [Reference Lynch23]. We are aware that Cryptosporidium oocysts can be present on raw meat at the retail level. C-EnterNet surveillance data illustrate that Cryptosporidium was isolated from raw retail pork chops, chicken breasts and ground beef purchased in Waterloo Region during the study period (4%, 4% and 19%, respectively) [5]. In this study, the data were insufficient to adequately explore foodborne transmission but suggest future work in this area.
In this study neither living on nor visiting a farm, petting zoo or fair were significant risk factors for disease. Nevertheless, direct contact with animals has been previously identified as an important risk factor for cryptosporidiosis. The disease has been reported in veterinary workers [Reference Preiser, Preiser and Madeo24, Reference Reif25] and people exposed to animals, as well as through visits to farms [Reference Kiang26–Reference Sayers29] and fairs or shows [Reference Bender30, Reference Ashbolt31]. In 2006, human infectious Cryptosporidium parvum (bovine genotype) was detected in both swine and dairy cattle manure (28% and 22% of samples, respectively) on local farms in Waterloo Region through the C-EnterNet program, illustrating its potential for spread to humans in contact with farms or farm areas, as well as transmission through the watershed.
Reported use of the municipal water supply was associated with increased odds of acquiring cryptosporidiosis, compared to a private water supply. Cryptosporidium has long been associated with contaminated drinking-water exposure. However, in this study, the community employs advanced water treatment of the potable water source (including filtration, ozonation, ultraviolet light radiation and chloramination). The risk of Cryptosporidium transmission from the municipal drinking-water source is a priori considered low. We suggest that this finding illustrates that urban residents (municipal water users) were more likely to acquire cryptosporidiosis than rural residents (private well-water users), and that water source was a surrogate for place of residence, rather than a risk factor for disease. To further explore this hypothesis, incorporating place of residence in the models would have been useful. However, residential addresses were not made available for this analysis. A recent cryptosporidiosis case-control study [Reference Lake32] found that urban place of residence was associated with an increased risk for cryptosporidiosis (particularly for C. hominis infections), which supports this hypothesis.
Person-to-person contact was associated with increased odds of disease in this study. Attending a social event during the exposure period was associated with a decrease in the odds of acquiring the disease, although this is probably a spurious finding, such that attending a social event was a significant exposure for the controls. Conversely, having a family member with a diarrhoeal illness was associated with increased odds of disease, suggesting the role that person-to-person transmission plays in these cases. The finding highlights the importance of good hand hygiene both in and out of the home. We did not explicitly ask about contact with children aged <5 years (or having a child attend daycare) in the case questionnaire, but this has been found to be a significant risk factor for cryptosporidiosis in both UK and USA studies [Reference Roy33, Reference Hunter34]. Because of its higher incidence in young children, Cryptosporidium can be easily transmitted in the home, nurseries or daycare settings and schools [Reference Hannah and Riordan35, Reference Cruikshank, Ashdown and Croese36]. A number of outbreaks have been associated with diapered infants and daycare attendance [Reference Turabelidze37]. A 2004 study found that children aged <4 years were more likely to be infected with C. parvum (35%) vs. C. hominis (20%) [Reference Hunter34], thus molecular typing of clinical cases in the study site might inform future interventions.
While travel-acquired cases were not included in this analysis, 25% of cases during the study period were associated with travel outside Canada. Travel has been recognized as a risk factor for cryptosporidiosis since the mid-1980s [Reference Khalakdina38], and is a significant source of Cryptosporidium infection in Waterloo Region.
STUDY DESIGN IMPLICATIONS
There are strengths and weaknesses to using an ill population as a control group. Previous studies using this design have proved useful for developing hypotheses about risk factors [Reference Ackman39–Reference Voetsch44]. Advantages of this study design include the probable reduction in differential recall (information) bias between cases and controls captured from the same surveillance system and the elimination of the need to enrol healthy controls, thereby reducing resources associated with study implementation.
Limitations of the modified case-control approach, based on sampling from a secondary rather than a primary base, include a systematic difference between the sample group and the true study base. However, this limitation is faced in traditional case-control studies as well, since the cases come from a secondary base and the controls come from a primary base. With the modified approach, it is also possible that some of the ill controls in this study may have had the same causal exposures as the cryptosporidiosis cases. Exposures leading to cryptosporidiosis and other factors associated with these exposures might thus be over-represented in the control group compared to the study base. This would result in a bias towards the null or even reversal of the relationship. Some studies have compared the use of a secondary-based ill control group with a primary-based population control group and have illustrated that the results do bias towards the null when the control group is comprised of individuals ill with a different disease [Reference Kist and Freitag41, Reference Voetsch44].
In this study, it is likely that some of the controls would have had similar exposures as the cases. This was explored in some detail, through the removal of giardiasis cases in the control group, based on the hypothesis that giardiasis and cryptosporidiosis cases might share similar exposures. By removing the giardiasis cases, the significance of the associations did not change, but the P values were reduced. However, as Rothman & Greenland explain [Reference Rothman and Greenland45], using a variety of diagnoses in the control group has the advantage of diluting the biasing effects of including a specific diagnostic group that is related to the exposure. Thus, we opted to take a conservative approach in our analysis and included all the non-cryptosporidiosis cases in the control group.
Finally, misclassification might also have occurred in this analysis if a stool specimen was contaminated with multiple pathogens and the correct aetiological agent was not identified. However, this would affect a traditional case-control study in the same manner.
In summary, the modified case-control design is one approach to develop hypotheses for identifying exposure sources of cryptosporidiosis. Restricting the analysis to endemic cases provides a mechanism for local public health practitioners to inform their case-finding and follow-up investigation skills, while supporting future research initiatives. Present findings support many of the a priori hypotheses of cryptosporidiosis transmission in North American communities, although further research is warranted.
CONCLUSION
This study has illustrated the importance of collecting standardized exposure information for every case of enteric illness in a community. It also demonstrated the use of a modified case-control design to refine exposure hypotheses. It has identified some significant transmission routes for the acquisition of a Cryptosporidium infection, including swimming in a river, drinking municipal water (urban residence) and having a family member with a diarrhoeal illness. The implications of these findings relate to two public health measures: continued advocacy for hand hygiene to prevent person-to-person transmission and the consideration of environmental exposures as a risk for cryptosporidiosis. Continued collection of enhanced, standardized exposure information for cases will enable the refining of current hypotheses specific to Waterloo Region. Molecular subtyping of all human clinical samples would help to inform our understanding of reservoirs and transmission of this pathogen.
ACKNOWLEDGEMENTS
The authors thank Barbara Marshall from the Public Health Agency of Canada, Nancy Sittler from the Waterloo Region public health authority, as well as the public health investigator staff, and the Toronto Region Public Health Laboratory for their involvement in the C-EnterNet surveillance programme. They acknowledge the statistical advice of William Sears in the Department of Population Medicine, Ontario Veterinary College, University of Guelph. Finally, they recognize Agriculture and Agri-Food Canada and the Public Health Agency of Canada for funding.
DECLARATION OF INTEREST
None.









