Article contents
Soil Improvement Following Addition of Chipped Wood from Twigs
Published online by Cambridge University Press: 30 October 2009
Abstract
Wood residues are applied to soils to improve their organic matter content and related biological, physical, and chemical properties. We monitored the changes in soil total C and N content, the bacterial, fungal and actinomycetal populations, and microbial biomass and activity during 20 weeks in the first season following the application of chipped wood from twigs (CWT), and as residual effects during the second growing season on a loamy soil (coarse loamy, mixed, frigid, Humic Fragiorthod) in Sainte-Brigitte-des-Saults (Québec) Canada. Wet-aggregate stability and the content of nutrients of the soil also were determined. Adding CWT stimulated the bacterial and actinomycetal populations very rapidly (within 8 weeks); in the second season the effect was less pronounced and gradually disappeared. The most significant and long-lasting effect was on the fungal population in two consecutive years of observation, with increases of up to 24-fold. This stimulation of fungi possibly was responsible for the large and significant increase in wet-aggregate stability observed in the second year. The effect of CWT on alkaline phosphatase activity and total C and N was observed only in the second season. Some 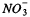 immobilization was seen only in the season immediately following residue application. The addition of the CWT also supplied micronutrients, in particular Zn, which would make it a useful source of some elements in deficient soils. Application of CWT to this soil greatly improved its quality, as revealed by its biological, chemical, and physical attributes.
immobilization was seen only in the season immediately following residue application. The addition of the CWT also supplied micronutrients, in particular Zn, which would make it a useful source of some elements in deficient soils. Application of CWT to this soil greatly improved its quality, as revealed by its biological, chemical, and physical attributes.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Cambridge University Press 1998
References
- 12
- Cited by




