In the Western world osteoporosis is an increasing problem, and its background is only partly known(Reference Woolf1). The modern Western diet has changed markedly by substituting SFA with PUFA, leading to a shifted composition of fatty acids (FA) with high levels of n-6 FA resulting in an increased ratio of n-6:n-3 FA(Reference Sanders2, Reference Ailhaud, Massiera and Weill3). The two families of essential FA (EFA), the n-6 and n-3 series, are represented by linoleic acid (LA; 18 : 2n-6) and α-linolenic acid (18 : 3n-3), which can be converted into long-chain PUFA of which the most important are arachidonic acid (AA; 20 : 4n-6), EPA (20 : 5n-3) and DHA (22 : 6n-3). These FA are of importance for many biological functions in the body.
During childhood and adolescence the bone is achieved by modelling and maintained by remodelling; thus the skeleton is under constant renewal. Bone modelling is a process of continuous growth, creating the bone shape. This process involves 100 % of the bone surfaces, while only 20 % of the surface is active at one time during the remodelling process. Although hereditary factors have been suggested as the most important for the individual bone mass, different nutrients have associations with bone mass(Reference Ilich and Kerstetter4). The influence of FA on bone has mainly been investigated in animals, indicating that EFA are associated with the development of normal bone mass(Reference Watkins, Lippman and Le Bouteiller5). Both n-6 and n-3 FA have been found to be related to bone metabolism in animal studies(Reference Watkins, Li and Lippman6). The n-6 FA influence bone resorption and accretion in a dose-dependent way and n-3 FA stimulate bone accretion(Reference Watkins, Li and Lippman6–Reference Korotkova, Ohlsson and Hanson8), affecting both trabecular and cortical bone, partly by influencing the concentration of AA, indicating the importance of a balanced diet for bone formation(Reference Watkins, Lippman and Le Bouteiller5). PGE2 and leucotrienes of the 4 series, major products of AA, have been shown to affect bone formation in a dose-dependent way; high levels stimulate bone resorption and low levels bone formation(Reference Watkins, Lippman and Le Bouteiller5, Reference Kruger and Horrobin9). Much of the effect of FA might thus be related to their eicosanoid products.
Dietary fat may modulate the formation of bone in children. Also cartilage is influenced by FA, since both LA and AA can reduce collagen synthesis while EPA has a stimulating effect(Reference Hankenson, Watkins and Schoenlein10). The balance might also be modulated by SFA, i.e. in chicks given butter fat the rate of bone formation was higher than in chicks given a diet high in n-6 FA(Reference Watkins, Shen and McMurtry11).
One study has shown correlations between dietary FA and bone mineral density (BMD) in healthy children(Reference Gunnes and Lehmann12) and a recent study has shown an association between serum concentrations of DHA and bone mass in adolescent males(Reference Hogstrom, Nordstrom and Nordstrom13). In cystic fibrosis a similar association between serum phospholipid FA and bone formation was shown in children and adolescents but not in adults(Reference Gronowitz, Mellstrom and Strandvik14, Reference Gronowitz, Lorentzon and Ohlsson15). In elderly and in patients after renal transplantation associations between BMD and FA have been reported(Reference Weiss, Barrett-Connor and von Muhlen16, Reference Baggio, Budakovic and Ferraro17).
The aim of the present study was to investigate if serum phospholipid FA, reflecting habitual dietary fat intake, were associated with BMD in a sample of healthy prepubertal children.
Subjects and methods
Subjects
Eighty-five healthy Caucasian children (fifty boys), aged 8·2 (sd 0·34) years, were investigated. They represented a subgroup, who accepted blood sampling, of ninety-six children for whom BMD data have recently been reported(Reference Eriksson, Mellström and Strandvik18). The children were representative of the general population of this age, except that the parents had higher education (49 % had university education compared with 22 % in the whole country). The parents were given local anaesthetic plasters (EMLA®; AstraZeneca AB, Södertälje, Sweden) to apply on the skin of the child 1 h before blood sampling.
The study was approved by the Ethics Committee at the University of Gothenburg (Gothenburg, Sweden) and performed in accordance with the Helsinki Declaration; informed consent was obtained from the parents and the children.
Anthropometric measurements
Height and body weight were measured using standardised equipment. BMI (kg/m2) and standard deviation scores (z-score) of height, weight(Reference Karlberg and Taranger19, Reference Wikland, Luo and Niklasson20) and BMI(Reference He, Albertsson-Wikland and Karlberg21) were calculated.
Dual-energy X-ray absorptiometry
The dual-energy X-ray absorptiometry (DXA) measurements were analysed using the Lunar DPX-IQ (GE Lunar Corp., Madison, WI, USA) as previously described(Reference Eriksson, Mellström and Strandvik18). Bone mineral content (BMC) of total body (TB), BMD of TB, lumbar spine L2-L4 (LS), total femur, and femoral neck and bone mineral apparent density of LS (volumetric BMD, bone mineral apparent density) were determined. The reference values obtained from the manufacturer were expressed in age- and sex-matched z-scores except for femur, for which no data were available.
Biochemical analyses
The blood samples were collected after an overnight fast on the same morning as the DXA measurements were performed. FA were analysed in serum phospholipids with capillary GLC as previously described(Reference Gronowitz, Mellstrom and Strandvik14). Serum Ca and 25-hydroxy-vitamin D (25(OH)D) were analysed according to routine (Sahlgrenska University Hospital, Gothenburg, Sweden).
Statistical analyses
Student's t test and Pearson correlation coefficients were used for continuous variables, which were approximately normally distributed. Statistically significant difference was set at P < 0·05 (two-sided test). Stepwise multiple regression analyses were used to analyse the relationship between BMD and anthropometry, serum Ca, vitamin D and different FA. Statistical analyses were performed with the statistical software program SPSS® 14.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Anthropometry
There were no significant differences between boys or girls, the averages being for height, 131 (sd 6) cm (z-score 0·4), weight 29 (sd 5) kg (z-score 0·7) and BMI 17 (sd 2) kg/m2 (z-score 0·4).
Bone measurements
The results of the DXA investigations are shown in Table 1. Boys generally had higher values of bone measurements than girls except for bone mineral apparent density in the LS; however, no difference was found between the sexes for BMDLS.
Table 1 Bone measurements in eighty-five healthy prepubertal children†
(Mean values and standard deviations)
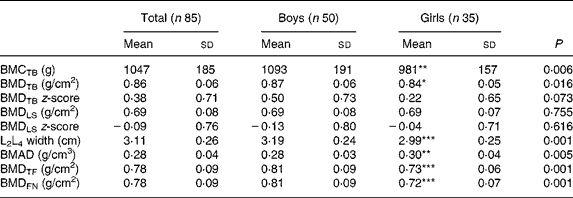
BMC, bone mineral content; TB, total body; BMD, bone mineral density; LS, lumbar spine; BMAD, bone mineral apparent density; TF, total femur; FN, femoral neck.
Mean value was significantly different from that of the boys: * P < 0·05, ** P < 0·01, *** P < 0·001.
† These children are included in a previous report(Reference Eriksson, Mellström and Strandvik18).
Serum phospholipid fatty acids
The mean serum phospholipid concentrations of the different FA are presented in Table 2.
Table 2 Concentration of major serum phospholipid fatty acids (molar %) in eighty-five healthy 8-year-old children
(Mean values and standard deviations)
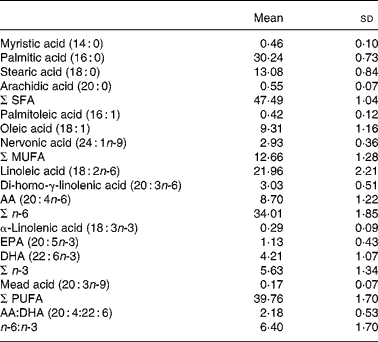
AA, arachidonic acid.
No sex difference was found. In correlation analysis nervonic acid (24 : 1n-9) and α-linolenic acid (18 : 3n-3) were significantly correlated with weight (r 0·266 (P = 0·014) and r − 0·224 (P = 0·040), respectively).
Biochemical analyses
Mean serum concentration of Ca was 2·48 (sd 0·06) mmol/l (reference values 2·20–2·60). Serum 25(OH)D averaged 70·5 (sd 27·3) nmol/l (reference values 25–162 nmol/l). Girls had significantly higher serum values of Ca and 25(OH)D than the boys (2·51 (sd 0·06) v. 2·46 (sd 0·05) mmol/l (P < 0·001) and 80·6 (sd 27·9) v. 63·5 (sd 24·8) nmol/l (P = 0·004), respectively). Serum Ca and vitamin D were only associated with bone mineral apparent density (r 0·267, P = 0·014 and r 0·269, P = 0·013, respectively), but the influence was not significant in the multiple regression analysis adjusted for FA, sex, height and weight. Vitamin D (25(OH)D) was negatively associated with stearic acid (18 : 0) (r − 0·292; P = 0·007) but positively with LA and EPA (r 0·213, P = 0·05 and r 0·221, P = 0·043, respectively).
Correlations between fatty acids and bone measures
The associations between bone parameters and FA are shown in Table 3. There was a weak but inverse relationship between palmitic (16 : 0) and stearic (18 : 0) FA and the bone parameters, and arachidic acid (20 : 0) was correlated with both BMD and bone mineral apparent density of the LS. Positive correlations were found for nervonic acid (24 : 1n-9) with bone measures of TB and all measurements of the hip. There was a general trend that LA was negatively associated with BMD, since that was found both for the LA concentration, the total n-6 concentration (which is mainly determined by LA) and the ratio of n-6:n-3 FA (Figs. 1 and 2). AA was positively correlated with BMCTB and BMDTB, a result supported by a positive correlation with the ratio of AA:LA (Fig. 3 (a) and (b)) and that the corresponding BMDTBz-score also was correlated with this ratio (r 0·346; P = 0·001). Furthermore the ratio between AA and its precursor di-homo-γ-linolenic acid (DGLA; 20 : 3n-6), an index of Δ5-desaturase activity, was positively correlated with BMCTB (r 0·222; P = 0·041), BMDTB (r 0·318; P = 0·003), the corresponding z-score (r 0·330; P = 0·002) and BMDtotal femur (r 0·229; P = 0·036).
Table 3 Correlation between major serum phospholipid fatty acids and bone parameters in eighty-five healthy 8-year-old children

BMC, bone mineral content; TB, total body; BMD, bone mineral density; LS, lumbar spine; BMAD, bone mineral apparent density; TF, total femur; FN, femoral neck.
* P < 0·05, ** P < 0·01.

Fig. 1 The associations between total body bone mineral density (BMDTB) and (a) the serum phospholipid concentration of linoleic acid (r − 0·251; P = 0·020) and (b) the ratio of n-6:n-3 fatty acids (r − 0·224; P = 0·039) in eighty-five healthy 8-year-old children.

Fig. 2 The associations between bone mineral density of the lumbar spine (BMDLS) and (a) the serum phospholipid concentrations of linoleic acid (r − 0·283; P = 0·009) and (b) total n-6 fatty acids (FA) (r − 0·286; P = 0·008) in eighty-five healthy 8-year-old children.

Fig. 3 The associations between total body bone mineral density (BMDTB) and (a) the serum phospholipid concentration of arachidonic acid (AA) (r 0·326; P = 0·002) and (b) the ratio of AA:linoleic acid (LA) (r 0·342; P = 0·001) in eighty-five healthy 8-year-old children.
Since the strongest relationships with serum FA concentrations were found in the LS, a multiple regression analysis was made adjusting for sex and anthropometric measures, known to influence bone. The multiple regression analysis showed that weight (P = 0·005) and arachidic acid (P = 0·023) were independently and positively associated with BMDLS. A weak but significant negative association was seen between α-linolenic acid and total BMC. No correlations were found between bone measurements and EPA, DHA, the ratio AA:EPA or AA:DHA. When adjusting for differences in body weight there was a significant correlation between DHA and BMDLS in the children with the highest body weight (>32 kg) and a mean BMI >19 kg/m2, representing the upper tertile of the children studied (Fig. 4).

Fig. 4 The associations between DHA and bone mineral density of the lumbar spine (BMDLS) in eighty-five healthy children, grouped by tertile of body weight: (○), tertiles 1 and 2 (r − 0·062; P = 0·636); (●), tertile 3 (body weight ≥ 32 kg) (r 0·417; P = 0·043).
Discussion
The present study shows that n-6 FA was similarly associated with bone mineralisation in humans as has been previously shown in animals(Reference Watkins, Li and Lippman6). There seemed to be a different influence of FA on BMD representing the TB and BMD representing the LS, which might indicate different relationships of FA to cortical and trabecular bone, since in the measurements of the TB the cortical bone predominates (Table 3). This different influence, also by different FA, has also previously been noticed in healthy children(Reference Gunnes and Lehmann12) and in children with cystic fibrosis(Reference Gronowitz, Mellstrom and Strandvik14).
In TB skeleton there was a strong positive correlation with AA and a weak negative correlation with LA and the ratio n-6:n-3. In the LS, where trabecular bone is more apparent, a negative correlation was found between the concentrations of LA and total n-6 FA in serum phospholipids and BMD and bone mineral apparent density, indicating that an excess of n-6 FA might predispose for lower BMD in the LS. A higher dietary ratio of n-6:n-3 FA has been found to be associated with lower BMD in the hip in women and in the spine of elderly women without hormone therapy(Reference Weiss, Barrett-Connor and von Muhlen16). In the LS there was no significant association with AA. These findings might be of strong importance, since it is during childhood that peak bone mass is achieved, determining the risk of osteoporosis later in life(Reference Heaney, Abrams and Dawson-Hughes22). The change of fat quality in the Western diet towards higher amounts of n-6 FA might in this context be of significant importance(Reference Sanders2, Reference Ailhaud, Massiera and Weill3).
Studies have indicated that PGE2 affects bone formation in a dose-dependent way, stimulatory at low levels and inhibitory at high levels, and therefore balanced levels of AA are important for bone formation(Reference Watkins, Shen and McMurtry11). Watkins et al. suggested that the concentration of AA is especially important in the young, since a moderate level of PGE2 showed a stimulatory effect on bone formation in the chicks, whereas a higher dose led to a decreased rate of bone formation(Reference Watkins, Shen and McMurtry11). An increased intake of n-6 FA would potentially increase AA and thereby PGE2 synthesis, which might further augment bone resorption. Another possibility is that very high concentrations of LA might inhibit Δ-6-desaturase leading to lower AA concentration as suggested by Sprecher(Reference Sprecher23). The positive association between the ratio of AA:LA and total bone mineralisation (Fig. 3 (b)) might support such interpretation. Since we could not in the present study estimate the eicosanoids, we cannot judge if the high LA was related to a high release of AA, stimulating the AA cascade. This might be an important focus for further studies.
FA pattern did not differ between boys and girls at this age. A correlation has been reported between dietary SFA and BMD, measured in the forearm with single photon absorptiometry in healthy children and young adolescents(Reference Gunnes and Lehmann12). A similar correlation between palmitic acid in serum phospholipids and BMD in the LS, that was found in our sample of healthy children, has also been reported in children with cystic fibrosis(Reference Gronowitz, Mellstrom and Strandvik14). A similar association was not found in healthy adolescent males(Reference Hogstrom, Nordstrom and Nordstrom13) or in adolescents and adults with cystic fibrosis(Reference Gronowitz, Mellstrom and Strandvik14, Reference Gronowitz, Lorentzon and Ohlsson15), suggesting that there might be age-related influences of FA on bone modelling.
In young men between the ages of 16 and 22 years, both positive associations of n-3 FA and BMD and a negative association of the ratio n-6:n-3 on the increment of BMDLS have been reported(Reference Hogstrom, Nordstrom and Nordstrom13). An inverse relationship between the AA:DHA ratio and BMDLSz-score has been reported in children but not in adults with cystic fibrosis(Reference Gronowitz, Mellstrom and Strandvik14) and DHA seemed to be connected to the modelling of bone since this association was the only one related to the final modelling in adolescents with cystic fibrosis(Reference Gronowitz, Lorentzon and Ohlsson15) and in healthy adolescents(Reference Hogstrom, Nordstrom and Nordstrom13). Such association might relate to our finding of an interaction between weight and DHA concentration on the BMD in the LS. The correlation between BMDLS and the DHA concentrations in serum was only significant in the biggest children, representing the highest tertile of weight and BMI (r 0·417; P = 0·043).
Studies in rats given isoenergetic diets only differing in the quality of EFA during pregnancy and lactation have shown that the bone in the adult offspring differed in relation to the EFA amounts or different ratios of n-6:n-3 supplied only during the perinatal period(Reference Korotkova, Ohlsson and Gabrielsson7, Reference Korotkova, Ohlsson and Hanson8). In these studies a balanced diet with a relatively high ratio of n-6:n-3 FA was associated with the highest BMD in the adult offspring(Reference Korotkova, Ohlsson and Hanson8), while a deficiency of both n-6 and n-3 FA was associated with decreased BMD in the adult animals(Reference Korotkova, Ohlsson and Gabrielsson7). This suggests a programming into adult life by the influence of FA. The findings indicated a possible influence on gene expression(Reference Jump24), which might by imprinting in early life regulate bone formation in the adults. That also raises the question if epigenetics by dietary factors have to be considered as risk factors for osteoporosis(Reference Waterland and Michels25), challenging the current view that bone mass is mainly determined by genes.
Vitamin D was only correlated to bone mineral apparent density but the influence disappeared in multiple regression analysis after adjusting for FA, sex, height and weight. The inverse correlations between vitamin D (25(OH)D) and stearic acid (18 : 0) on the one hand, and LA and EPA on the other, might illustrate the associations between vitamin D and food intake.
In summary, the present study of healthy prepubertal children shows that the n-6 FA, linoleic acid and AA, in serum correlated with BMD in an opposite pattern, more expressed as a difference impact on BMD of the TB and the LS. It also suggests that an overload of n-6 FA was associated with lower BMD. Also influences of SFA were suggested. It would be most interesting if FA could be determined in the bone tissue, since it is well known that serum concentrations of FA are not congruent with all other tissues.
An association between FA and bone parameters during growth as reported in the animal experimental literature(Reference Watkins, Li and Lippman6) is corroborated by the present study. The results indicate a complex interaction of different FA, which might be associated with different modelling of the skeleton. This is suggested by the specific association between DHA and bone growth during adolescence as previously reported(Reference Hogstrom, Nordstrom and Nordstrom13, Reference Gronowitz, Lorentzon and Ohlsson15) and supported by the association found in the biggest children in the present study. Also the associations between bone density and SFA found by us and others(Reference Gunnes and Lehmann12), and the dose-dependent relationship between LA and its longer derivative, AA, precursor to PGE2, which has been indicated as one major modulator of bone growth in animals(Reference Watkins, Shen and McMurtry11), were corroborated in the present study. We found no associations with EPA or the AA:EPA ratio although animal studies have indicated their importance for bone formation by modulating PGE2 synthesis(Reference Watkins, Li and Lippman6). The results have to be confirmed in larger and longitudinal studies to find if there is a dynamic influence of different FA on the skeletal development and if there are long-term effects by imprinting as indicated by the animal studies.
Acknowledgements
The present study was supported by grants from the Paediatric Research Foundation at the Queen Silvia Children's Hospital, the Swedish Nutrition Foundation, the Solstickan Foundation and the Swedish Mayflower Charity Foundation for Children.
Käthe Strandner, Kerstin Herlitz, Senada Catic and Berit Holmberg are gratefully acknowledged for technical assistance. The authors thank Kirsten Mehlig for valuable statistical assistance.
The authors' responsibilities were as follows: B. S. conceived the idea for the study and was together with S. E. responsible for the design. S. E. collected the material and performed the statistical analysis. D. M. was responsible for the DXA measures. S. E. drafted the paper and all authors reviewed the report critically and approved the final version.
None of the authors had personal or financial conflict of interest.











