INTRODUCTION
National surveillance of invasive pneumococcal disease (IPD) in New Zealand is laboratory based, and provides data on the epidemiology of the disease, distribution of serotypes and antimicrobial susceptibility [Reference Green and Cawley1–Reference Brett and Martin4]. These data contribute to the development of policies to prevent and treat pneumococcal disease, including immunization policy and guidelines for empirical therapy.
The 7-valent pneumococcal conjugate vaccine (PCV-7) is currently only included in the publicly funded childhood immunization schedule for very high-risk infants. Extension of its use to all infants is being considered as part of a revised schedule due for implementation in 2008. The 23-valent pneumococcal polysaccharide vaccine (PPV-23) is recommended, but not publicly funded, for people at risk including all those aged ⩾65 years [5].
The serotype distribution and antimicrobial susceptibility of invasive pneumococci in New Zealand was last reviewed for the 1995–1997 period [Reference Brett and Martin4]. In this paper, we describe aspects of the epidemiology of IPD, the serotypes involved, and antimicrobial resistance for the 8-year period 1998–2005.
METHODS
All diagnostic laboratories in New Zealand were requested to refer all invasive isolates of Streptococcus pneumoniae (i.e. isolates from blood, CSF and other normally sterile sites) to the national reference laboratory at the Institute of Environmental Science and Research (ESR). Patients from whom such isolates were received were defined as cases of IPD. Information received with each isolate included the case's name, age and sex. Clinical details were often not provided or were not useful. Only the first isolate from a case was included in the analyses.
Serological typing was performed by the capsular antigen reaction (Neufeld test) using the Danish system of nomenclature and sera obtained from the Statens Seruminstitut [Reference Lund, Henrichsen, Bergan and Norris6]. Penicillin and cefotaxime susceptibilities were determined by Etest (AB Biodisk, Solna, Sweden), using Mueller–Hinton agar with 5% sheep blood and incubation for 16–20 h in 5% CO2, and interpreted according to the 2006 CLSI standards [7]. The meningitis interpretive standard was used to interpret the cefotaxime minimum inhibitory concentrations (MICs). Penicillin and cefotaxime ‘non-susceptibility’ includes resistance and intermediate resistance. Chloramphenicol, co-trimoxazole, erythromycin, tetracycline and vancomycin susceptibilities were determined by the CLSI disc susceptibility testing method [7, 8]. Multiresistance was defined as resistance to three antibiotics in addition to penicillin resistance or non-susceptibility. The full antimicrobial susceptibility of the isolates from two cases was not determined and these isolates were excluded from the susceptibility analyses.
Data on the age and sex distribution of the New Zealand population for each of the years from 1998 to 2005 were obtained from Statistics New Zealand. Statistical analyses were performed with SAS software v. 9.1.2 (SAS Institute Inc., Cary, NC, USA). The χ2 test or Fisher's exact test, as appropriate, were used to determine the significance of any observed differences. Poisson regression analysis was used to determine whether there were significant trends over time. An associated P value ⩽0·05 was used to identify whether a difference or trend was significant.
RESULTS
Between 1998 and 2005, S. pneumoniae isolates from 3903 cases of IPD were referred to ESR from throughout New Zealand. There was no significant change in the incidence of IPD during the 8-year period. The annual incidence averaged 12·4/100 000 and varied from a minimum of 9·9 in 1998 to a maximum of 13·8 in 2001. The rate in the latest year (2005) was 12·0/100 000.
Most (92·8%) isolates were cultured from blood, 5·1% from CSF and 2·1% from other normally sterile sites, including joint and ascitic fluid aspirates.
Incidence by age and gender
Age was not known for three cases and gender was not known for another three cases. Nearly one-third (31·4%) of IPD cases were children aged <5 years and 32·7% of cases were aged ⩾65 years. The incidence was highest in infants <2 years and adults ⩾75 years (Fig. 1). The average annual incidence rate in infants <2 years was 100·8/100 000 and in children <5 years the rate was 54·2/100 000. The incidence was higher in males in all age groups except the 55–64 years group. This age and gender distribution was consistent throughout the 1998–2005 period.
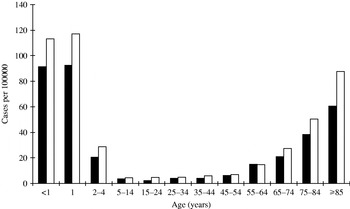
Fig. 1. Average annual incidence of invasive pneumococcal disease by age and gender (■, female; □, male), 1998–2005.
Capsular types
Serotypes 14, 6B, 4, 19F, 9V, 23F and 18C were the most common serotypes, with 14 by far the most common (Table 1). These seven serotypes consistently ranked among the ten most frequent serotypes each year. However, during the 8-year period there were significant changes in the prevalence of several of the serotypes, with serotypes 14, 6B and 4 increasing, and serotypes 9V, 7F, 1 and 12F decreasing.
Table 1. Most prevalent serotypes among isolates from cases of invasive pneumococcal disease, 1998–2005
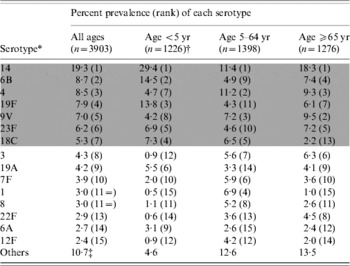
The shaded area denotes those serotypes included in PCV-7.
* The specific serotypes listed are those that accounted for ⩾2% of all cases.
† The distribution of serotypes in cases aged <2 years was very similar to that in the <5 years age group.
‡ Includes 18 (0·5%) isolates that could not be typed.
Potential coverage of IPD by PCV-7 and PPV-23
The proportion of IPD cases that would potentially be covered by the serotypes in the two pneumococcal vaccines currently licensed for use in New Zealand, the Wyeth PCV-7 (Prevenar®) and the MSD PPV-23 (Pneumovax®), is shown in Table 2. The serotype coverage of PCV-7 has varied over the years. Among infants aged <2 years, coverage ranged from a low of 69·4% in 1998 to a high of 91·5% in 2002. Among children aged <5 years, the lowest coverage was 71·6% in 1998 and the highest 88·3% in 2002.
Table 2. Potential coverage of PCV-7 and PPV-23, based on the serotypes causing invasive pneumococcal disease, 1998–2005
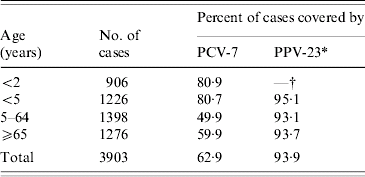
PCV-7 includes types 4, 6B, 9V, 14, 18C, 19F and 23F.
PPV-23 includes types 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F.
* Serogroup 15 isolates were not serotyped due to their small numbers (13). For the calculation of PPV-23 coverage, the serogroup 15 isolates were assumed to be covered.
† Vaccine not licensed for use in infants aged <2 years.
Antimicrobial susceptibility
Over the whole 8-year period, there was no significant change in the prevalence of penicillin resistance or penicillin non-susceptibility (Table 3). However, penicillin resistance decreased significantly between 1998 and 2002, and then increased during the 2003–2005 period. Cefotaxime resistance, cefotaxime non-susceptibility, erythromycin resistance and tetracycline resistance all increased significantly. Penicillin and erythromycin co-resistance and cefotaxime and erythromycin co-resistance also increased. Co-trimoxazole resistance was consistently 30–40%. All isolates were susceptible to vancomycin.
Table 3. Antimicrobial resistance among isolates from cases of invasive pneumococcal disease, 1998–2005
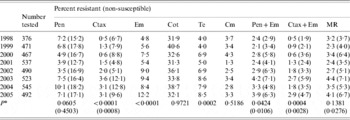
Pen, Penicillin; Ctax, cefotaxime; Em, erythromycin; Cot, co-trimoxazole; Te, tetracycline; Cm, chloramphenicol; MR, resistant (non-susceptible) to penicillin and resistant to at least three other antibiotics.
*P value from Poisson regression analysis for trend of increasing or decreasing resistance between 1998 and 2005.
Among the penicillin-resistant isolates, 30·9% were also cefotaxime resistant, 91·6% were cefotaxime non-susceptible, 47·4% were erythromycin resistant, and 51·0% were multiresistant. The most common pattern of multiresistance among the penicillin-resistant isolates was cefotaxime, co-trimoxazole, erythromycin and tetracycline resistance. Among the penicillin non-susceptible isolates, 52·3% were also cefotaxime non-susceptible, 31·5% were erythromycin resistant, and 32·2% were multiresistant.
Antimicrobial resistance was more common among isolates from IPD cases <5 years old and those aged ⩾65 years (Fig. 2). Resistance to all antibiotics and multiresistance were significantly more prevalent among isolates from cases <5 years old compared with isolates from other cases. When isolates from cases aged <5 years were compared with only those from cases ⩾65 years, only resistance to co-trimoxazole, erythromycin and tetracycline, and multiresistance were more prevalent.
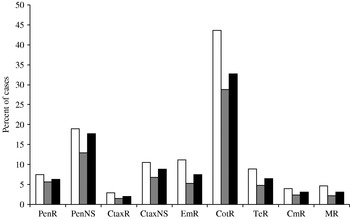
Fig. 2. Antimicrobial resistance by case's age (□, <5 years; ![]() , 5–64 years; ■, ⩾65 years), 1998–2005. Abbreviations: PenR, penicillin resistant; PenNS, penicillin non-susceptible; CtaxR, cefotaxime resistant; CtaxNS, cefotaxime non-susceptible; EmR, erythromycin resistant; CotR, co-trimoxazole resistant; TeR, tetracycline resistant; CmR, chloramphenicol resistant; MR, multiresistant to penicillin and at least three other antibiotics.
, 5–64 years; ■, ⩾65 years), 1998–2005. Abbreviations: PenR, penicillin resistant; PenNS, penicillin non-susceptible; CtaxR, cefotaxime resistant; CtaxNS, cefotaxime non-susceptible; EmR, erythromycin resistant; CotR, co-trimoxazole resistant; TeR, tetracycline resistant; CmR, chloramphenicol resistant; MR, multiresistant to penicillin and at least three other antibiotics.
Four serotypes, 19F, 9V, 23F and 14, accounted for ⩾87·3% of the invasive penicillin-resistant pneumococci every year between 1998 and 2005, and 93·2% over the whole 8-year period (Table 4). Serotype 9V, which was not associated with multiresistance, was the prevalent penicillin-resistant type in the 1998–1999 period. It was surpassed by serotype 19F during the 2000–2005 period. Most (93·0%) penicillin-resistant serotype 19F isolates were multiresistant, with cefotaxime, co-trimoxazole, erythromycin and tetracycline resistance the most common pattern. Two-thirds (67·9%) of these multiresistant serotype 19F isolates had cefotaxime MICs of ⩾4 mg/l. Of the other two prevalent penicillin-resistant serotypes, 23F was usually multiresistant but not cefotaxime resistant, whereas serotype 14 was not usually multiresistant.
Table 4. Most prevalent serotypes among penicillin- and cefotaxime-resistant and non-susceptible isolates from cases of invasive pneumococcal disease, 1998–2005
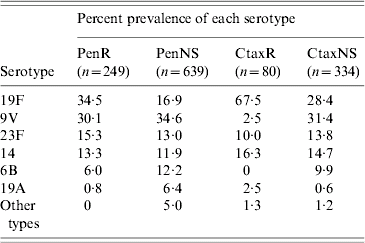
PenR, Penicillin resistant; PenNS, penicillin non-susceptible; CtaxR cefotaxime resistant; CtaxNS cefotaxime non-susceptible.
All but two (99·2%) of the penicillin-resistant isolates were serotypes included in PCV-7 and all were one of the PPV-23 serotypes. All of the penicillin-resistant isolates from IPD cases aged <5 years were serotypes included in PCV-7. Among the penicillin non-susceptible isolates, 88·9% were serotypes included in PCV-7 and 96·2% were one of the PPV-23 serotypes. Among the penicillin non-susceptible isolates, 87·0% from cases aged <5 years were serotypes included in PCV-7 and 96·5% from cases aged ⩾65 years were one of the PPV-23 serotypes.
DISCUSSION
The average annual incidence of IPD in New Zealand over the last 8 years (12·4/100 000) is consistent with reports from other developed countries [Reference Fedson, Musher, Plotkin and Orenstein9]. IPD occurs predominantly in infants aged <2 years and adults ⩾75 years, and is more common in males than females. The incidence in <2-year-olds found in this study (101/100 000) and <5-year-olds (54/100 000) is very similar to that recorded in these two age groups during a 1984–1992 paediatric study in the Auckland region of New Zealand (110 and 56/100 000, respectively) [Reference Voss10].
This predominance of disease in the very young and elderly is typical for a population in the absence of infant immunization with pneumococcal conjugate vaccine [11]. Although PCV-7 has been available on the private market in New Zealand since 2001, less than 200 doses have been sold in any one year (L. Mackie, Wyeth). Similarly, while PPV-23 is recommended for people at risk, including all ⩾65-year-olds, it is not publicly funded and uptake is also poor [Reference Drinkovic12].
Unfortunately, it is not possible to describe ethnicity or other demographic risk factors for IPD because this information was usually not reported with the referred isolates. In New Zealand, people of Maori and Pacific Island ethnicities generally suffer disproportionately high rates of many infectious diseases [13]. In the earlier Auckland-based study, the incidence of IPD among Pacific Island children was nearly four times, and that among Maori over twice, the rate in other ethnic groups [Reference Voss10].
The serotypes causing most of IPD in New Zealand are consistent with international reports [Reference Watson14–Reference Denham and Clarke16]. Specifically, 80·9% of IPD in infants aged <2 years is caused by serotypes included in PCV-7. Data emerging from the United States, where PCV-7 was introduced into the infant immunization programme in 2000, indicate that the conjugate vaccine confers herd immunity with a reduction in IPD in unvaccinated older children and adults [Reference Reingold17]. Our data show that, compared with cases aged <5 years, there was a poorer match (~50–60%) between the serotypes causing disease in older cases and the types in PCV-7. It will therefore be interesting to observe the degree to which IPD is prevented in older persons if routine infant PCV-7 immunization is introduced.
An increase in IPD caused by serotypes not contained in PCV-7 has been observed following introduction of the vaccine. In particular, increases in disease caused by serotype 19A have been reported [Reference Beall18–Reference Kyaw20]. Serotype 19A is already a common cause of IPD in the <5 years age group in this country and therefore it will be important to continue to monitor the seroepidemiology of IPD should routine infant PCV-7 immunization be introduced.
This study also provides insight into rates and patterns of antibiotic resistance among pneumococci causing invasive disease in New Zealand, particularly in relation to serotypes. Penicillin resistance was uncommon among invasive pneumococci prior to 1995 and thereafter began to increase [Reference Martin and Brett3, Reference Brett and Martin4]. By 1998 penicillin resistance had reached 7·2% and non-susceptibility 15·2%. There has been little overall change in penicillin resistance and non-susceptibility since then. However, there were fluctuations in the prevalence of penicillin resistance in spite of relatively constant non-susceptibility. In contrast, cefotaxime resistance and non-susceptibility, penicillin and erythromycin co-resistance, and cefotaxime and erythromycin co-resistance all increased between 1998 and 2005.
Stabilization and even decreases in penicillin resistance have recently been reported in other countries, including Europe [21], the United States [Reference Waterer22], and Taiwan [Reference Hsueh23]. However, while penicillin resistance may have stabilized in New Zealand it is still relatively high. Compared with 10·1% penicillin resistance in New Zealand in 2004, the United Kingdom reported <1% and Australia 4·4% for the same year. The comparative data for penicillin non-susceptibility in each country were 18·2%, 3% and 13·5%, respectively [21, Reference Roche24].
Antibiotic resistance in pneumococci is often reported to be a greater problem in children aged <5 years [Reference Vanderkooi25]. This is also the case in New Zealand where resistance to all antibiotics and multiresistance was highest among isolates from IPD cases <5 years old. While there were regional differences (data not shown), the national rate of 18·9% penicillin non-susceptibility among invasive pneumococci from children aged <5 years means that children with presumed pneumococcal meningitis should not receive penicillin alone as first-line empiric treatment, and consideration should be given to including vancomycin until culture and susceptibility results are available.
The changing patterns of resistance over time and the differences between age groups can, to some extent, be explained by changes in serotypes. Four serotypes, 19F, 9V, 23F and 14, have consistently accounted for about 90% of all invasive penicillin-resistant pneumococci in New Zealand. Serotype 9V was the most prevalent penicillin-resistant type in 1998 and 1999, after which time 19F became the most prevalent. Most of the penicillin-resistant serotype 19F isolates were notable for their multiresistance and high-level cefotaxime resistance (MIC ⩾4 mg/l). High-level cefotaxime resistance in serotype 19F pneumococci emerged in New Zealand in 1997 among both invasive and non-invasive pneumococci [Reference Brett26]. The majority of multiresistant serotype 19F pneumococci with high-level cefotaxime resistance in New Zealand have been shown to be a single-allele variant (ST271, aroE 15→4) of the Taiwan19F-14 clone [Reference Bean and Klena27].
Substantial decreases in the incidence of antibiotic-resistant IPD have been reported following the introduction of PCV-7 into the routine childhood immunization schedule in the United States [Reference Kyaw20, Reference Stephens28]. These decreases have occurred in the target vaccination age group as well as older children and adults, albeit not as dramatically. Globally and in New Zealand, most antibiotic-resistant pneumococci are serotypes 6B, 9V, 14, 19F or 23F – all serotypes included in PCV-7. Our results indicate that all of the IPD in children aged <5 years caused by penicillin-resistant isolates would be covered by the serotypes included in PCV-7.
This study summarizes the results of ongoing laboratory-based surveillance of IPD in an era prior to the introduction of routine infant pneumococcal immunization. The data are useful for determining rates of culture-proven IPD, serotype distribution and antimicrobial susceptibility. A more comprehensive understanding of the epidemiology of IPD in New Zealand would be available if the disease was made notifiable to Medical Officers of Health. This will be particularly important to measure the effects of any changes in immunization policy and practice.
ACKNOWLEDGEMENTS
National laboratory-based surveillance of invasive pneumococcal disease is funded by the New Zealand Ministry of Health. We thank laboratory staff throughout New Zealand for referring isolates and Alokananda Maitra, ESR, for assistance with statistical analyses.
DECLARATION OF INTEREST
None.










