INTRODUCTION
The Chikungunya virus (CHIKV), a member of the Alphavirus genus in the Togaviridae family [Reference Porterfield and Schlesinger1] was first isolated in Tanganyka (Tanzania) in 1952 [Reference Lumsden2]. The virus has since been isolated in numerous countries in Eastern, Western and Southern Africa, and Asia [Reference Hammon3]. Chikungunya disease in humans is characterized by fever, headache, nausea, vomiting, myalgia, cutaneous rash and arthralgia [Reference Jupp and Monath4].
Since 1952, CHIKV has caused many outbreaks, and since 2005, the virus has been circulating and isolated in India and in the islands of the south-western Indian Ocean including Comoros, Mayotte, Seychelles, Réunion, Mauritius and Madagascar (http://www.invs.sante.fr). In Réunion Island, the first documented case was in a patient returning from Comoros in March 2005 [Reference Paquet5]. Re-emergence of this virus in this part of the world was unpredictable because consecutive epidemics had occurred at least 7–8 years apart. In July 2006, the number of cumulated cases was estimated to reach around 260 000 and the peak incidence was observed during the second week of February 2006 with 45 000 new cases. In Réunion Island the vector was identified as Aedes albopictus.
The Groupe Hospitalier Sud Réunion (GHSR) is a 1300-bed hospital with facilities that are equivalent to similarly sized hospitals in mainland France. The GHSR is located in the south (population 300 000), where the epidemic started, and diagnosed the first Chikungunya case in the island. The GHSR maternity ward is the largest in France (4500 deliveries per year) and observed the first cases of materno-foetal CHIKV transmission [Reference Robillard6]. Neurology, neurosurgery and neuroradiology are also important GHSR departments where the first cases of encephalopathy caused by CHIKV were diagnosed. Confronted with Chikungunya disease complications that were unknown to the international medical community, we organized the present survey to achieve a better knowledge of the virus in a short period of time and with little funding. A questionnaire-based survey among the GHSR staff, well aware of the importance of such study, was chosen as the best means to achieve fast and low-cost despatching and data collecting. First, the questionnaire inquired about the clinical symptoms. Considering the clinical signs had been poorly described in the literature, we were interested in comparing clinic-based diagnoses and serologically confirmed CHIKV infections. In order to identify potential clues that may be useful for the management of future cases, we also investigated the medical prescriptions and traditional medicines, the individual use of anti-vector protection, and the participants' environmental factors that might impact on viral transmission.
MATERIAL AND METHOD
Participants
The GHSR administrative and health-care staff members were all invited to participate in the study.
Data collection
The questionnaire was made available to all staff members, in a printed version or on the intranet web, on 1 April 2006. The questionnaire was anonymous and contained multiple-choice questions to be answered by ticking the appropriate boxes. It was dispatched with the assistance of GHSR, after approval by the scientific and ethical committee.
The first page of the questionnaire asked for information about the participant (age, gender, position within the hospital staff, home address, preventive means used against the insect vector, declaration whether he/she considered having contracted Chikungunya disease) and about his/her household (age, gender, Chikungunya declaration). The participants declared whether they did not (negative) or did (positive) contract the disease at any time between February 2005 (beginning of the epidemic) and the day they completed the questionnaire. The positive declaration was based either on the participant's own observation of clinical signs, or a medical diagnosis with or without serological confirmation. In cases where a household member had contracted the disease, we recorded the date when the first symptoms had developed, duration of arthralgia, and if/when symptoms had developed again after recovery.
The second page of the questionnaire focused on participants who declared having contracted the disease. We recorded the date of the first clinical signs, symptoms, serological confirmation, therapy used, occurrence of relapses (clinical signs and pain intensity), medical follow-up and professional consequences.
The major clinical signs of CHIKV infections reported in the questionnaire were fever and arthralgia with or without cutaneous rash. Arthralgia was considered chronic after 4 weeks of evolution between the date the first symptoms appeared and the date the questionnaire was completed.
Data analysis
The information gathered from the questionnaire was entered into an Access database (Microsoft) and data analysis was performed using Access queries. Mean and standard deviation (s.d.) values were calculated using the Excel software (Microsoft). Statistical analyses were performed using the χ2 test with Yates' correction. The results were considered significant for a 95% confidence interval (95% CI) (P<0·05).
RESULTS
General data in studied populations
At the end of April we collected 567 questionnaires (P group), representing a response rate of 23·8% of the GHSR staff. This responding population was not randomly drawn and therefore may not be representative of the southern island population. There were more female participants (66%). The participants' mean age was 40·9±10·2 years (range 20–64) and mean age was similar for both genders (female 40·2±9·9, range 20–62; male 42·3±10·2, range 23–64). The GHSR staff was distributed as follows: 8·8% technical, 21·4% administrative, 16·7% medical and 53·1% paramedical.
The participants and their household members totalled 1745 people (H group). The H group was structured as follows: female 51·4%, male 48·6%, mean age 30·0±39·6 years (range 0–98). The 0–20 years age group was under-represented compared to the island's total population (28·8% vs. 53%), whereas the 40–59 years group was over-represented (35% vs. 11·5%). However, the proportions of 20–39 and >60 years age groups were similar to those in the island's total population (32·4% vs. 32% and 4·1% v. 3·7%). The H group represented 0·78% of the 222 200 inhabitants in the surveyed districts. The less represented districts were Saint Philippe (0·2%) and Saint Leu (0·27%). Values ranged from 0·56% (Saint Louis) to 1·5% (Etang Salé) for the other districts.
General proportion of CHIKV infection per inhabitant
In the P group 221 participants declared having contracted Chikungunya disease (putative cases: P+ group=38·97% of participants, 95% CI 34·9–43·0), 71 (32·1%) of which were confirmed by serology (confirmed cases). The majority of cases in the P+ group (72·9%) were recorded between 1 January and 1 April 2006.
In the H group 613 out of 1745 people surveyed declared having contracted the disease (H+ group: 35·1%, 95% CI 32·8–37·4).
General proportion of CHIKV infection per house
There was at least one positive inhabitant per house in 58·4% of the surveyed houses, including 33·2% houses where all inhabitants considered they had contracted the disease. The mean proportion of inhabitants per house that considered themselves to have been infected by CHIKV was 63·1%.
Factors influencing the proportion of CHIKV infection
Gender
In the P and H groups, the proportion of CHIKV infections was significantly higher in females (P 43%, H 38·8%) than in males (P 31%, H 31·2%) [P group: P=0·007, relative risk (RR) 1·37, 95% CI 1·08–1·75; H group: P=0·0009, RR 1·24, 95% CI 1·24–1·41].
Age
The proportion in each 20-year age band in the H group showed a significant increase (3 d.f., P=0·00001; age group 0–20 years 25%, 21–40 years 33·4%, 41–60 years 42·5%, >60 years 40·4%). The χ2 test revealed a significant difference between age groups 0–20 and 21–40 years (P=0·02) as well as between the 21–40 and 41–60 years age groups (P=0·009). A non-significant difference was found between age groups 41–60 and >60 years.
Within the 0–20 years age group the proportion was 18% for ages 0–10 years and 32·2% for ages 11–20 years (P=0·005).
Garden
In the P group 90·6% of households had a garden. The presence of a garden seemed a risk factor, as 36·2% of inhabitants who owned a garden declared they contracted the disease, against 22·4% of inhabitants in houses with no garden (P=0·001, RR 1·62, 95% CI 1·17–2·23).
Moreover, the percentage of houses where at least one inhabitant considered themself as having been CHIKV-infected was greater where there was a garden (60·1%) vs. no garden (41·5%) (P=0·01, RR 1·45, 95% CI 1·04–2·01).
Place of residence
The proportion of CHIKV-positive participants varied between districts (Fig.). The lowest proportion was observed in Le Tampon (n=391, 16·9%) and the highest in the districts of Saint Louis [n=212, 54·7%, P<10−5, RR 0·31 (when compared to Le Tampon), 95% CI 0·31–0·40], Saint Joseph (n=166, 57·8%, P<10−5, RR 0·29, 95% CI 0·23–0·38) and Saint Philippe (n=12, 58·3%, P=0·04, RR 0·41, 95% CI 0·20–0·82). The second lowest, yet still significant proportion was in Petite Ile (n=97, 28·9%, P=0·007, RR 0·58, 95% CI 0·40–0·86). The proportion among the ⩾20-year-olds was 61·6% in Saint Louis, 61·4% in Saint Joseph and 18·5% in Le Tampon.
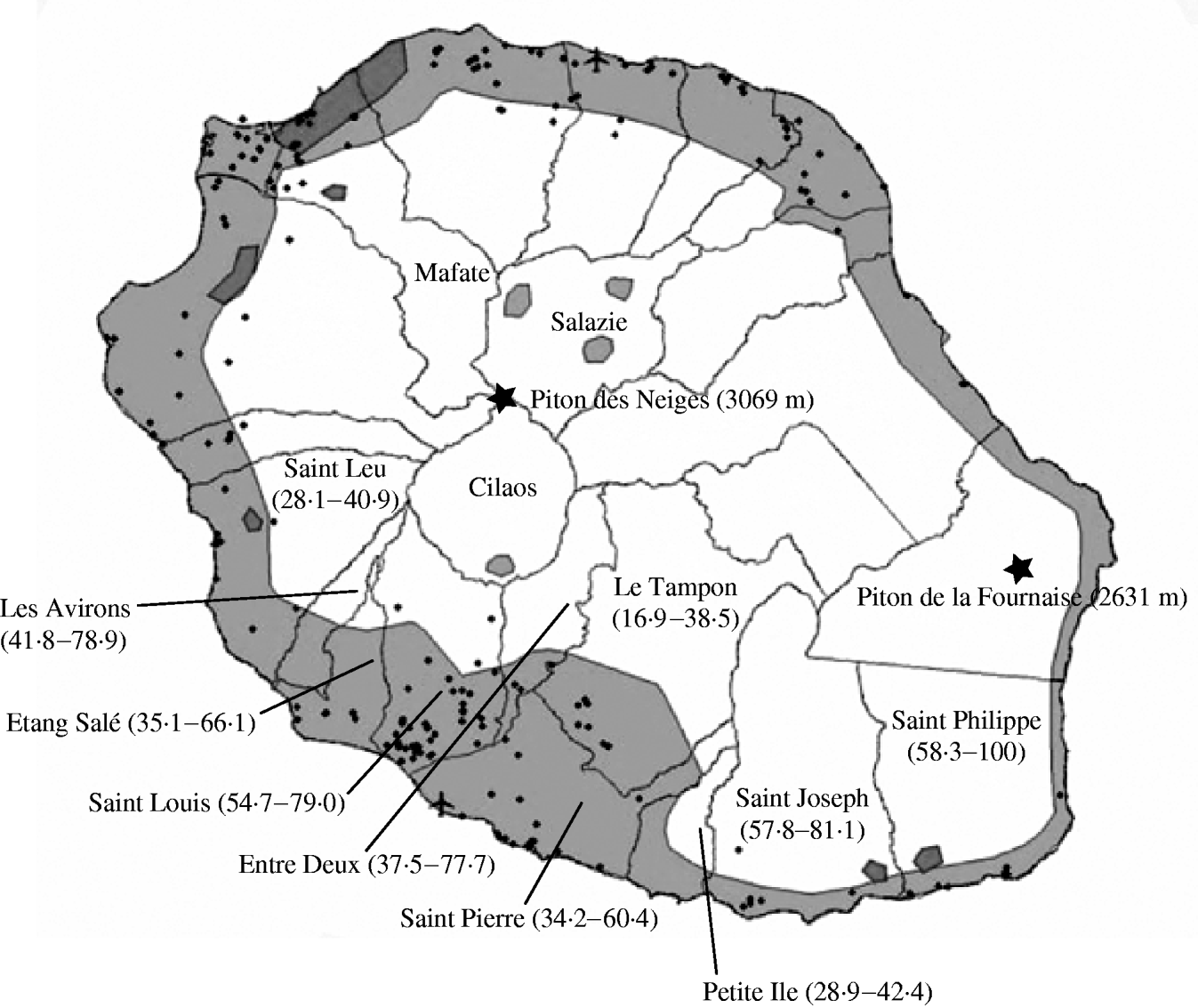
Fig. Chikungunya proportion in the different districts of Réunion Island based on survey results. For each district the figures indicate the disease proportion per inhabitant and the percentage of houses where at least one inhabitant contracted the disease. Modified from the 2003–2004 Aedes spp. distribution map by DRASS (Direction Régionale des Affaires Sanitaires et Sociales) (www.acreunion.fr/hygieneetsecurite/chik/pdf/DiapoDRASS.pdf). ![]() , Aedes albopictus distribution;
, Aedes albopictus distribution; ![]() , Aedes aegypti distribution; ●, A. albopictus breeding sites.
, Aedes aegypti distribution; ●, A. albopictus breeding sites.
Individual preventive means
In our study the use of insecticide appliances in the house did not significantly decrease the number of CHIKV-diseased people (use of insecticide 35·5% vs. no use 31·9%, P=0·41), nor did it decrease the number of houses where at least one inhabitant contracted the disease (use of insecticide 59·2% vs. no use 50·0%, P=0·24).
The use of skin insect repellent (69·7%) did not significantly decrease the number of diseased people in the P group although the P value was close to the significance threshold (221 positive participants: use of repellent=35·2% vs. no use=47·6%, P=0·08). However, the difference becomes significant when excluding the month of February 2006 when viral transmission was the highest (145 positive participants: use of repellent=26·8% vs. no use=36·8%, P=0·027).
Clinical symptoms
The nature and localization of Chikungunya clinical symptoms were recorded for the P+ group (Table 1). Arthralgia and fever were the signs that were chosen during the epidemic to diagnose a CHIKV infection and were recorded in 90·5% of the cases. Only nine participants declared having contracted the disease without developing any arthralgia. For three of them, CHIKV infection was serologically confirmed. The remaining six were not confirmed by serology but had consulted a physician. They had headache, asthenia, myalgia and joint swelling. Digestive and cutaneous signs were recorded in over 80% of cases. For each symptom group (Table 1) there was no significant difference between medical (n=24) and non-medical staff, between serologically confirmed and unconfirmed CHIKV infections, and between CHIKV-infected participants who were confirmed by a medical diagnosis and participants who self-diagnosed the disease (P>0·05 for each comparison, data not shown). Statistical analysis showed no significant difference in clinical signs between gender except for swellings which occurred more frequently in women than men (88·1% vs. 68·2%, P=0·003). As far as invalidating forms of the disease were concerned (walking and prehensile difficulties), no significant difference was found between participants above or below 40 years of age.
Table 1. Nature and localization of the clinical signs recorded for 221 surveyed participants who contracted Chikungunya disease

Disease evolution
In the P+ group, only 76 participants (34·4%) recovered without any relapse or clinical symptoms persisting longer than a month. On the contrary, clinical symptoms persisted for 22 participants (9·9%) including 0·45% for 5 months, 1·35% for 4 months, 1·35% for 3 months, 4·50% for 2 months, and 2·25% for 1 month. Further, six participants (2·7%) were hospitalized, including five women and one man whose ages ranged from 25 to 55 years. These six developed fever, myalgia, asthenia and invalidating arthralgia (walking and prehensile difficulties); four had node enlargement; five reported cutaneous symptoms; four developed a haemorrhage and five a neurological involvement.
In the P+ group 123 participants (55·6%) had relapses. The mean number of relapses was 2·1±1·2 and the mean time interval between recovery and relapse was 4·2±3·9 weeks (range 1–32). Clinical signs during relapses were arthralgia (96·7% of cases), oedema (61·0%), fever (18·7%), and cutaneous symptoms (5·7%). Joint pain was felt more intensively in relapses for 31·4% of cases, identical in 36·4%, and less intense for 32·2% of cases.
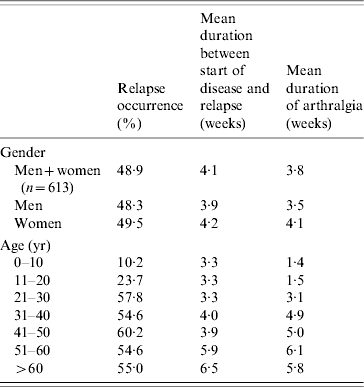
In the H+ group 48·9% relapsed at least once (Table 2). The percentage of relapses was not significantly different between men and women (P=0·75). Relapse frequency was very low in <10-year-olds (10·2%), and null in <5-year-olds. However, relapses were more frequent in 10- to 20-year-olds. The mean time interval between primary infection and relapse was 4·1 weeks. The mean duration of joint pain during relapses was 3·7 weeks, increasing with age (1·3 weeks for the 0–10 years group, 6·1 weeks for the 51–60 years group). The percentage of relapses in the H+ group did not differ significantly from that in the P+ group, similar to H+ group participants who received a corticosteroid treatment vs. those who did not.
Table 2. Relapse occurrence in relation to gender and age
Treatments
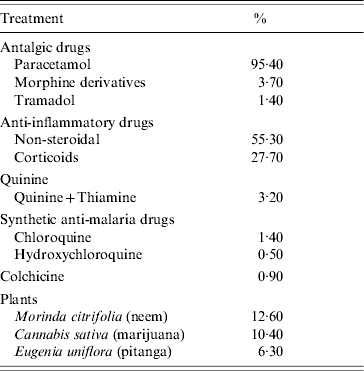
In the P+ group 98·2% of participants reported a medical prescription. The main treatments are listed in Table 3.
Table 3. Record of the main treatments used by 221 surveyed participants who contracted Chikungunya disease
Drugs
Paracetamol was used by 95·4% of P+ group members. Corticoids were prescribed to 27·7% of patients to treat arthralgia, in particular for the invalidating forms (31·5% vs. not invalidating 12·9%, P=0·007). Some patients who took paracetamol also used morphine derivatives (3·6%, data not shown). Similarly, some patients were prescribed both corticosteroids and non-steroidal anti-inflammatory drugs (18·3%, data not shown).
Natural medicine
Herbal medicine was used by 40·7% of P+ group. Three major plant species were reported: Morinda citrifolia (neem, noni), Cannabis sativa (marijuana, zamal) and Eugenia uniflora (pitanga) (Table 3), plus numerous others: Aloe vera (aloes), Terminalia bentzoë (benjoin), Fernelia spp. (buis), Cinnamomum burmannii (cinnamon), Cymbopogon citratus (lemon grass), Eucalyptus spp., Lantana camara (galabert), Pelargonium×asterum (geranium), Panax ginseng (ginseng), Musseandra landia (quinquina), Felipendula ulmara (reine des prés), Lippia citriodorata (lemon verbena). Five people (2·2%) also took propolis.
Physiotherapy
In the P+ group 13·1% consulted a physiotherapist. More P+ participants with relapse used physiotherapy (16·3%) although the percentage increase was not significant (P=0·17). The situation was similar for P+ participants who had walking difficulties (17·6%, P=0·23).
Sick leave
In the P+ group 85·5% consulted a physician and 76·8% went on sick leave. The mean sick leave duration was 9·9±8·7 days (range 1–60 days).
DISCUSSION
Population sample
In our study we defined our positive and negative cases based on the participant's declaration of whether they had/had not contracted a CHIKV infection. Our definition is valid because for each symptom group we found no significant difference between positive participants who were confirmed by a medical diagnosis and positive participants who self-diagnosed the disease. Similarly, there was no significant difference between the proven (serologically confirmed) and the putative cases. The clinical signs have a good positive predictive value and hence are sufficiently good criteria for the diagnosis of CHIKV infections during the epidemic's high transmission phase. Diagnoses based either on the participants' declaration or medical consultation are, therefore, reliable and convenient to study the epidemiology of CHIKV.
Statistical analysis
The present study was organized in an outbreak situation and aimed at urgently addressing simple issues, as a quick understanding of the epidemic was required. At the time the questionnaire was introduced we lacked sufficient knowledge of the expected data structure to apply complex statistical methods such as multivariate analyses [Reference Robillard6, Reference Le Bras7]. In addition, we knew that the participating population may not be representative of the southern population of Réunion Island and that the recruited population might not be sufficient to draw definitive conclusions. Consequently, the χ2 test with Yates' correction was chosen for data analysis.
Proportion of Chikungunya disease-positive participants
Our population sample, selected in although not representative of the southern part of Réunion Island, revealed a Chikungunya disease proportion of 35·1%, based on participants' declarations, with 72·9% of cases between 1 January and 1 April 2006. On 30 March 2006, the DDASS (Direction Régionale des Affaires Sanitaires et Sociales) estimated that 230 000 people (30·7% of Réunion population) had contracted the disease to date. The proportion was highest in several southern districts, particularly Saint Pierre, Saint Philippe, Entre Deux, Etang Salé and Les Avirons [8].
The disease proportion in our study was higher in women than men. This was also observed during a DRASS study. The observed difference is possibly due to men and women being differently exposed to the insect vector by their activities and their environment. We observed a similar situation in a previous study of leptospirosis incidence, where women were mainly contaminated by the canicola serovar, and men by the icterohaemorraghiae serovar, each serovar occurring in different environments [Reference Duval9].
The proportion of CHIKV infections also varied with age, also possibly because different age groups are exposed to different environments and have different immune responses to infections. The young (aged <20 years), which represent more than half of the Réunion Island population (53% in 2002), were the least affected by the disease. DRASS obtained similar results. Few children aged between 0 and 10 years were affected possibly because their parents ensured they were well protected.
Most people have a garden (90·6%) in Réunion Island. From our results, presence of a garden may constitute a risk factor for CHIKV infection.
The Chikungunya disease proportion varied significantly between districts. Although our population sample is not representative of the island's southern population, the distribution of cases per district correlates with the distribution of Aedes albopictus in Réunion Island. A. albopictus population distribution depends on temperature and rainfall and thus varies with seasons and altitude. Consequently, the insect vector is essentially present on the coastline and in the three central caldeiras; Cilaos, Salazie and Mafate [Reference Hamon10]. Réunion population is dense (299 inhabitants/km2) although very sparse over half of the island because of its topography. The areas of high population density match those where A. albopictus occurs, favouring the contact between population and vector.
The districts where GHSR staff members live are all located on the coastline with the exception of Le Tampon which has an altitude ranging between 400 m and 1600 m. Le Tampon showed the lowest Chikungunya proportion (16·9%). The highest proportion values were found in Saint Joseph and Saint Philippe, located at the extreme south of the island and therefore exposed to greater rainfall.
In our study, 72·9% of people who contracted the disease were infected between January and March 2006: this correlates not only with climatic factors, but also possibly with the emergence of a virus variant better adapted to A. albopictus in September 2005 [Reference Schuffenecker11].
Means of prevention
People have been attempting to protect themselves against A. albopictus bites during the epidemic. In our study mosquito coils or electrical appliances inside the house did not seem to prevent CHIKV infections as we found no significant difference between people who used and those that did not use this type of protection. Similarly, there was no significant difference between people who applied skin insect repellent and those that did not, although the P value was close to the significant threshold. The apparent inefficacy of skin insect repellent may be explained by the extremely high exposition (45 000 cases per week and for 750 000 inhabitants, as estimated at the peak of the epidemic) resulting in a high probability for virus transmission to occur at the slightest neglect in using prevention. In the questionnaire we did not ask whether the repellent was applied according to the manufacturer's instructions. Considering that the difference between those who used and those that did not use repellent becomes significant when excluding the period of highest transmission (February 2006), we suggest that these products are probably efficient in preventing CHIKV infections, but only when applied correctly. Further analyses with a representative population sample are needed to confirm these results.
Vaccination would be the best prevention. A CHIKV vaccine was produced and tested: it was well tolerated and induced the production of neutralizing antibodies for 85% of the vaccinated test population a year after injection [Reference Edelman12]. However, the Asian CHIKV strain used to produce the vaccine is not closely related to the Réunion Island strain, as revealed by sequence data analysis [Reference Schuffenecker11].
Clinical symptoms
During the epidemic fever in Tanzania in 1952–1953, Robinson described in detail for the first time the clinical symptoms that were subsequently known under the term Chikungunya [Reference Robinson13] (meaning ‘that which bends up’ in Sawhili). The disease is also named buka-buka (broken-broken) by the Kinshasa people from the invalidating joint pain that it causes [Reference Muyembe-Tamfum14]. The disease is characterized by sudden arthralgia and often strong fever [Reference Robinson13, Reference Fourie15, Reference Kennedy16]. These main symptoms are associated with headaches and skin rashes. The latter vary in nature (morbilliform, maculopapular or purpuric rash), in localization (face, chest, limb extensor surfaces), in frequency (from 50% to 80%), and develop at various times after the symptoms first appear. Other symptoms include digestive disorders such as anorexia, dysgeusia, diarrhoea, nausea and vomiting. The participants in our study developed characteristic symptoms during the epidemic. Vesicular dermatosis also occurred, and although they were infrequent and atypical of Chikungunya they had been previously reported in Ross River virus infections [Reference Rulli17].
Some of the people surveyed might have been considered as unaffected by Chikungunya, as asymptomatic or paucisymptomatic forms were revealed during seroprevalence studies [Reference Thonnon18]. On the contrary, some participants were confirmed by serology as having contracted the disease but did not suffer from arthralgia even though this symptom was considered essential for the participant to be included in our study. Osterrieth et al. also reported clinical signs that did not include arthralgia [Reference Osterrieth19]. Clinical signs varied in Chikungunya epidemic records from different parts of the world: for instance, digestive disorders were not reported in Thailand. The clinical signs described in Réunion Island are similar to those in Africa, and possibly correspond to a clinical form of the disease related to a genome variant of the virus.
Relapses
During the epidemic several people recovered then again developed the clinical symptoms of Chikungunya. In 1955, Robinson recorded a relapse case with joint pain but no fever, where no other cause was identified for the illness. Relapses occurred intermittently in most cases, and in some cases up to 4 months after primary infection [Reference Robinson13]. The present survey enabled some characteristics for the relapses to be identified, which have rarely been described to date. Relapses were frequent (almost 50% of cases) and affected men and women equally. Relapse occurrence increased with age: no relapse was recorded in the 0–5 years group, while the relapse rate did not significantly differ between age groups >20 years. Age appeared to be a factor influencing both relapse and chronic forms of the disease [Reference Fourie15]. The time interval between recovery from primary infection and first relapse was about 4 weeks and increased with age, as did the mean duration of joint pain during relapses, although we did not identify the cause. The mean number of relapses per relapsing patient was 2·1. However, as our survey was carried out in April 2006 this figure will need to be updated.
Chikungunya relapses are essentially characterized by joint pain and oedema. When pain intensity during relapses is compared to that during primary infections it is felt less by one third of participants, identical by another third and stronger by the remaining third.
Several hypotheses may explain the relapses. A possible re-inoculation with the virus from a mosquito bite seems unlikely, as there were patients who relapsed after they left the epidemic area. A possible re-activation of the virus was suggested, however, relapsing patients tested RT–PCR negative. The hypothesis of virus particles subsisting within connective tissues near joints (periostal and endostal bones, tendons) or synovial tissue was also raised. This was experimentally demonstrated for Sindbis virus and Ross River virus infections, respectively [Reference Heise20, Reference Soden21]. Alternatively, the virus may persist inside the macrophage cells, as was demonstrated for Ross River virus [Reference Rulli17]. Viral reactivation may explain why 18·7% of people who relapsed had fever, although fever might result from an immunity illness. Relapses may also be subsequent to an inflammatory mechanism as we observed acute synovial inflammation in some cases. Consequently, relapses might be chronic forms with weak, hardly detectable inflammatory phases.
Importantly, relapses were not subsequent to a corticoid treatment, as they occurred in patients who were not prescribed corticoids. Further, we did not find a significant increase in relapses for patients who were treated with corticoids.
Treatments
As the clinical signs varied, most CHIKV-infected people opted for antalgic drugs. Paracetamol was the most used (95·4% of treatments) and was frequently associated with non-steroidal anti-inflammatory drugs (55·3%). The wide use of paracetamol may be the cause for severe liver afflictions that were diagnosed during the epidemic, particularly when doses >3 g/day were taken. Morphine was seldom used although it was effective during the initial phase of the disease [Reference Robinson13]. Corticoids were also prescribed to treat arthralgia (27·7% of cases), particularly invalidating forms. Synthetic anti-malaria drugs were rarely prescribed possibly because they are theoretically not adapted for the disease. Chloroquine is, however, effective in treating chronic arthralgia most probably due to its anti-inflammatory properties that are adapted for the treatment of some auto-immune illnesses [Reference Brighton22]. Chloroquine might also be useful during the viraemic phase because the molecule inhibits viral replication [Reference Savarino23] like the α-interferon ribavirin association [Reference Briolant24]. The effectiveness of chloroquine in treating CHIKV infections during viraemia is currently under trial in Réunion Island.
Plants are an integral part of the Réunion Island pharmacopeia and therefore were mainly used during the outbreak to treat fever, pain and inflammation. Some plant species such as Fernelia spp. are also known for their antiviral properties. The efficacy of these plants or association of plants has not been studied, particularly for the hepatotoxic risk they might present when taken with paracetamol.
Career consequences
Three out of four P+ participants went on sick leave for about 10 days due to Chikungunya illness, hindering patient care during a period of increasing activity [Reference Staikowsky25].
Prospects
CHIKV transmission was high from January to April and reached 45 000 cases per week in February 2006. Isolated cases or epidemiologic centres were still observed during the 2006 austral winter. If vertical transmission exists, it may have contributed to maintaining a low level of virus transmission during the austral winter, the insect vector currently being the only known reservoir in Réunion Island other than man. The isolated cases observed during the austral winter suggest that the epidemic might develop again during the 2006–2007 austral summer. However, our results indicate that over 60% of the Réunion Island population that is most exposed to CHIKV infection (aged ⩾20 years, living on the coastline) have developed immunity against the virus. In addition, in one third of households, all inhabitants have been infected and are therefore immune; mosquito larvae eradication programmes are being carried out; anti-mosquito and anti-larvae control measures are still being advertised to the population. This suggests that the next epidemic would not be as major as in 2006, provided the insect vector geographic distribution does not change to reach less immune populations living at high altitude.
ACKNOWLEDGEMENTS
The authors thank Mr A. Lysandre, Director of GHSR, for making the questionnaire available to the hospital staff, Mrs F. Michault for data entry in Access, and Mrs S. Bonari (IUT Saint Pierre, Réunion Island), Mrs C. Dalban (CIC-EC INSERM, Saint Pierre, Réunion Island) and Dr M. Lecuit (Pasteur Institute, Paris) for critical reading of the manuscript.
DECLARATION OF INTEREST
None.








