INTRODUCTION
Cryptosporidiosis, a disease caused by the parasitic protozoon Cryptosporidium, is among the infectious diseases that must be reported in the Czech Republic. However, with the exception of several sporadic cases just after the commencement of reporting in the mid-1980s, few cases and no outbreaks have been reported. Currently, the low awareness of Czech physicians about cryptosporidiosis leads to few laboratory tests for Cryptosporidium in patients suffering diarrhoea. Of ~100 000 stool specimens analysed for ova and parasites in 2003, only 1300 were analysed for Cryptosporidium, and only seven (0·5%) specimens were found to be positive. However, the group of 1300 specimens came from people suspected to have cryptosporidiosis (e.g. when other common agents were not confirmed). Similar statistics were reported in 2001 and 2002. Since 1997, only 21 cases of cryptosporidiosis have been reported; 38% occurred in travellers to Central America or Vietnam and 48% were in children. Cryptosporidium was the primary diagnosis for 11% of 198 children (aged 2–36 months) admitted to the hospital for diarrhoea in one region of the Czech Republic between 1992 and 1996 [Reference Chmelik1].
Limitations in diagnosing and reporting Cryptosporidium infections might have resulted in the low levels of reported cases [Reference Frost, Calderon and Craun2]. There is a chain of events that affects whether an infected person appears as a reported case [Reference Frost, Craun and Calderon3]. The infected person must have symptoms and seek medical care. Before reporting a case, the physician must request the proper test, the patient must provide a stool sample, and the laboratory must be capable of conducting the test with high proficiency. Many cases may not be recognized because the symptoms are mild, no stool specimen was requested, or the laboratory test was inadequate. Other factors may also influence the reporting of cryptosporidiosis in the Czech Republic. For comparison, in neighbouring Germany where the reporting of cryptosporidiosis has been mandatory since 2001, some 1000 cases have been reported annually [4, 5]. The rate of reported cases in Germany is ~20-fold higher (4 vs. 0·2/1 million persons per year).
A number of waterborne cryptosporidiosis outbreaks have been reported in the United States and the United Kingdom [Reference Craun6, Reference Craun, Calderon, Craun and Pontius7]. Drinking water is known to be a significant source of Cryptosporidium transmission, often in the absence of memorable illness [Reference Frost8]. In the United States, oocysts have been detected in a number of water sources and drinking-water systems, including filtered water with low turbidity levels [Reference Aboytes9]. During 1997–2002 in the Czech Republic, the Water & Environmental Technology Team conducted Cryptosporidium analysis of 100 water samples collected for several research projects and at the request of drinking- water managers. Oocysts were found in 50 (79%) raw water samples and 18 (49%) treated drinking-water samples. These water-quality data are similar to results in the United States and Japan [Reference Hashimotoa, Kunikaneb and Hirata10, Reference Lechevallier, Norton and Lee11]. However, no outbreaks of cryptosporidiosis have been reported in the Czech Republic. The public health significance of finding oocysts in drinking-water sources and systems is not clear. Detected oocysts may not be infective for humans. Even if infectious, routine exposure of populations to low concentrations of oocysts may increase levels of protective immunity that reduces symptomatic illness [Reference Frost12, Reference Frost13].
Cryptosporidium infection elicits a serological response in most infected humans, and surveys of various populations have estimated the prevalence in populations intentionally or unintentionally exposed to oocysts [Reference Moss14–Reference Egorov22]. Serological studies have focused on IgG serological responses to the 15/17-kDa and 27-kDa antigen groups. Responses to these two markers appear to be specific for Cryptosporidium infection. Infection usually elicits a serological response to these antigen groups that peaks 4–6 weeks after infection [Reference Moss14–Reference Muller16]. The 15/17-kDa marker declines to baseline levels observed prior to the infection in 4–6 months after infection while the 27-kDa marker remains elevated for 6–12 months [Reference Muller16]. Studies have found that drinking-water source (ground vs. surface) and/or water-treatment procedures (with or without filtration) are related to the detection and the density of oocysts in treated drinking water [Reference Aboytes9–Reference Lechevallier, Norton and Lee11] as well as the serological response [Reference Frost8, Reference Frost19–Reference Frost25]. Recent studies also suggest that strong serological responses (⩾20% of the positive control), possibly resulting from low-dose waterborne and/or foodborne oocyst exposure, may confer protective immunity to cryptosporidiosis [Reference Frost13].
We investigated the seroprevalence of the 15/17-kDa and 27-kDa antigen groups in persons residing in four areas of the Czech Republic with different sources of drinking water or water treatment. We selected serum samples from 200 persons who had participated in a larger, previously conducted serological survey. The primary purpose of the current study was to determine whether the seroprevalence of these markers was associated with source-water and treatment characteristics.
METHODS
Source of the sera
The sera samples were selected from the Serum Bank of the Centre for Epidemiology and Microbiology at the National Institute of Public Health. The Serum Bank serves as a depository for the sera collected from multi-purpose serological surveys, which have been performed in the Czech Republic annually from 1960 to 1990. Since 1990, surveys have been conducted every 4 years. These surveys provide information about the immunity status of the population and serve as the basis for decisions regarding changes of existing vaccination programmes and the source of data for specialized epidemiological investigations. Each survey collects several thousand serum samples from a representative population sample of different age groups from various regions of the country. Blood samples are taken from healthy individuals not suffering from any fever and showing no signs of immunodeficiency. Every blood sample is coded and recorded with essential epidemiological data.
Study sites
In selecting the serum samples for this study, we evaluated the numbers of samples that were available from several geographic areas with an unequivocally defined source of drinking water, especially riverbank infiltration. From sera collected in September and October 1985, we were able to select 50 samples from each of four areas that use drinking water from clearly defined sources: (1) riverbank infiltration, (2) wells in a fractured sandstone aquifer, or (3) two different surface waters. Based on our previous studies in other countries, we felt that this number of sera samples was adequate to detect possible seroprevalence differences among the areas. An additional 14 duplicate samples were included for laboratory quality control. In each area, samples were selected from the following age groups: 20–29 years (24 samples); 30–39 years (14 samples); and 40–49 years (12 samples). The male:female ratio of persons selected was 1:1 in each water-source and age group. The sera were analysed early in 2004. In the selected areas, no waterborne outbreaks or cryptosporidiosis outbreaks were reported in the years 1984–1986. The following describes the water sources and treatment for the four areas in 1985:
Site A
is a city (33 000 population) located in southern Bohemia. During the study period the city obtained water from an open reservoir and partly from a nearby river, both with water of rather poor quality. The water was treated by chemical coagulation, rapid sand filtration, and chlorine disinfection.
Site B
is a larger, predominantly industrial city (175 000 population) located in western Bohemia. Water was obtained from a river that has a vast catchment area mostly agricultural with some forestland. Water was treated by chemical coagulation, settling, rapid sand filtration, and chloramine disinfection.
Site C
is located in central Bohemia and includes three cities (~20 000 population in each city). Groundwater from 40–50 wells was collected and chlorinated at a single location before distribution to the three cities. The wells (40–60 m deep) were drilled into a fissured, sandstone aquifer, and the groundwater is considered to be of good quality. Occasionally, coliform bacteria have been detected in untreated well-water samples.
Site D
is a city (28 000 population) in southern Moravia. Several vertical wells collected water after riverbank infiltration through an unconfined aquifer. The water was treated by rapid sand filtration with subsequent chlorination.
Western blot procedures
Sera were analyzed by immunoblot to measure IgG serological response to the 15/17- and 27-kDa Cryptosporidium antigen groups. The analytical methods and control procedures have been described elsewhere [Reference Frost8, Reference Frost20–Reference Frost26]. The intensity of the serological responses to each antigen group was digitally analysed by an IS-2000 Digital Imaging System (Alpha Innotech, San Leandro, CA, USA) that calculates the pixel density of the manually selected band of the immunoblot. The intensity of each band is standardized by comparing the response intensity of the unknown sample to the response intensity of a positive Cryptosporidium control serum. A standard set of sera from persons with laboratory-confirmed infections served as the positive control sample for our analysis of the 15/17 and 27-kDa antigen groups. The initial control sera were obtained from the Centers for Disease Control (Atlanta, GA, USA), and subsequent control sera were mixed to obtain serological responses similar to the initial control sera. Having a standard control sera provides comparable positive control intensity for all of our studies and thus, allows comparison of findings between studies. For analysis purposes, we categorized the imaged serological responses as non-detectable, detectable with a response of <20% of the positive control (weak response), and ⩾20% of the positive control (strong response). Quality control procedures include the comparison of the intensity of response for a duplicate positive control and a duplicate randomly selected unknown sera sample for each blot [26]. The blots were repeated when the replicate analyses differed. One pair of samples was deleted because of concerns that these samples were mislabelled, and only 49 samples from site D were included in the study. Analysis of variance (ANOVA) was conducted on mean responses and differences in the per cent responses that were strong using a χ2 test.
RESULTS
Eighty per cent of all subjects had a detectable response to the 15/17-kDa antigen (57, 71, 90 and 96% by site) and 94% had a detectable response to the 27-kDa antigen (86, 94, 98 and 98% by site). For the 199 study participants, the respective mean responses to the 15/17-kDa and 27-kDa antigens were 43% and 41% of the positive control response. The mean responses to each antigen differed by site (P<0·0001) (Table 1).
Table 1. Mean Cryptosporidium antigen responses for persons in study
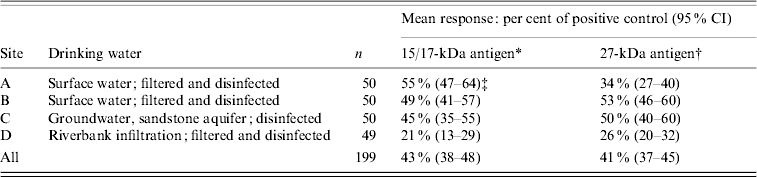
* ANOVA, F value=10·7, P<0·0001 (3 d.f.).
† ANOVA, F value=11·3, P<0·0001 (3 d.f.).
‡ For example, the mean response to the 15/17-kDa antigen was 55% of the positive control response.
The mean response for each antigen was lowest in site D, which is supplied with riverbank infiltration water (P<0·001). The mean responses to the 15/17-kDa antigen for sites A, B, and C were more than twice the mean response in site D. Differences were less pronounced for responses to the 27-kDa antigen. Comparing sites A, B and C after excluding site D, we found no statistically significant differences in mean responses to the 15/17-kDa antigen group by site, but there were statistically significant differences by site in the mean response to the 27-kDa antigen (P=0·001) with a mean response of 34% for site A vs. 53% for site B and 50% for site C.
Because a previous study in the United States [Reference Muller16] found that strong responses (⩾20% of the positive control) to the 15/17-kDa antigen were related to a reduced risk of diarrhoeal and gastrointestinal illness, we examined the distribution of strong responses by site (Table 2). Strong responses also differed by site for both the 15/17-kDa (P<0·0001) and 27-kDa antigen (P<0·01). The per cent of persons who had a strong response to the 15/17-kDa antigen ranged from 72% to 84% for the sites that did not use riverbank infiltration water compared to 33% for people using riverbank infiltrated water. The per cent of persons who had a strong response to the 27-kDa antigen ranged from 68% to 88% for sites that did not use riverbank infiltration compared to 57% for people using riverbank infiltration. After adjusting for age and gender in a logistic regression, the riverbank infiltration population was significantly less likely than the other population groups to have a strong response to either the 15/17-kDa (OR 0·14, 95% CI 0·07–0·27) or 27-kDa antigen (OR 0·38, 95% CI 0·20–0·76).
Table 2. Intensity of responses to Cryptosporidium antigen: per cent of sera samples (95% CI) with a response ⩾20% of the positive control

* Positivity ⩾20% of positive control; χ2=37·2 (P<0·0001, 3 d.f.).
† Positivity ⩾20% of positive control; χ2=13·0 (P<0·01, 3 d.f.).
‡ For example, 80% of persons had sera with a high response defined as ⩾20% of positive control.
DISCUSSION
Of the 199 persons in our survey, 67% had a strong response to the 15/17-kDa antigen and 72% had a strong response to the 27-kDa antigen. We compared these findings to other populations. Only 31–45% of people surveyed in two cites in Australia had a strong response to either antigen; both cities used surface water sources [Reference Frost, Hunter, Waite and Ronchi27]. In a city in Russia where surface water was used, 61% of persons had a strong response to the 15/17-kDa antigen and 66% had a strong response to the 27-kDa antigen [Reference Frost, Hunter, Waite and Ronchi27]. In a city in Italy where surface water was used, 66% of persons had a strong response to the 15/17 antigen and 35% had a strong response to the 27-kDa antigen [Reference Frost, Hunter, Waite and Ronchi27].
Surveys in ten cities in the United States and Canada reported a wide range of responses. Depending on the water source, from 13% to 66% of the persons studied had a strong response to either the 15/17- or 27-kDa antigen [Reference Frost, Hunter, Waite and Ronchi27]. Serological studies in four paired cities, one with surface water and the other with groundwater sources, found that the surface-water cities had a higher percentage of persons with a strong response to either antigen [Reference Frost, Hunter, Waite and Ronchi27]. In the four surface-water cities, 22–82% more persons had a high response to the 15/17-kDa antigen and 21–35% more persons had a high response to the 27-kDa antigen. For example, in two central Midwestern US cities, 31% of the people in the surface-water city had a high response to the 15/17-kDa antigen compared to only 17% in the groundwater city [Reference Frost, Hunter, Waite and Ronchi27].
In our study, only 33% of the people using riverbank infiltration had a strong serological response to the 15/17-kDa antigen. If we presume that a strong serological response not only reflects infection but also confers protective immunity, then 67% of the riverbank population would remain susceptible to cryptosporidiosis. However, in the other three sites that are more typical of drinking-water sources and treatment in the Czech Republic, only 16–21% of the population remain susceptible. With only a small fraction of this population at elevated risk of illness from infection, it is unlikely that a cryptosporidiosis outbreak would be detected, even with optimal disease surveillance. The difficulty in detecting a cryptosporidiosis outbreak is supported by two waterborne outbreak investigations where illnesses in visitors alerted officials to a possible outbreak. In outbreaks in Oregon [Reference Frost28] and Ontario, Canada [Reference Frost29], town residents, routinely exposed to waterborne oocysts, appeared to be immune to symptomatic cryptosporidiosis whereas visitors suffered considerable illness. Serological studies in both locations found a high prevalence of strong responses to the 15/17-kDa antigen in town residents.
Chappell et al. [Reference Chappell30] provide additional evidence of protective immunity in a study of C. parvum infectivity and clinical outcomes in 17 healthy adult volunteers who had pre-existing serum IgG to parasite antigens. The subjects were challenged with a single dose of 500–50 000 oocysts, a level of exposure that exceeds the usual oocyst levels in water [Reference Chappell30]. Chappell et al. [Reference Chappell30] concluded that ‘a significant portion of individuals may experience protection from infection in situations where low oocyst concentrations are present’. Infection in this study was defined as the presence of oocysts in the stool or symptoms compatible with cryptosporidiosis. In a previous clinical study, Okhuysen et al. [Reference Okhuysen31] found a serum IgG response in 32% of 19 previously infected human volunteers who were re-challenged with 500 oocysts 1 year later [Reference Chappell30]. In this earlier study [Reference Okhuysen31], fewer volunteers shed oocysts during the second exposure but the rate of diarrhoea was similar to that seen by Chappell et al. [Reference Chappell30]. Moss et al. [Reference Moss15] also found evidence of protective immunity; volunteers with strong baseline serological response to the 27-kDa antigen group were at significantly lower risk of cryptosporidiosis. Exposures to Cryptosporidium in most drinking waters are relatively low but may be frequent, and thus, drinking-water exposures may offer protection against outbreaks of cryptosporidiosis [Reference Frost12, Reference Frost13].
It is apparent that the persons in our survey have come in contact with Cryptosporidia much more frequently than is suggested by the number of clinically confirmed and reported cases of cryptosporidiosis. We do not know whether the course of this infection in the Czech Republic leads primarily to asymptomatic cases or is self-limiting with mild illness. Although it is possible that some of the symptomatic cases are classified and reported as diarrhoea and acute gastroenteritis of unknown but suspected infectious origin (diagnosis A09 ICD-10), the infections identified here are not among the reported cases of cryptosporidiosis in the Czech Republic. The serological responses found in our survey suggest that a large fraction of the study population had increased Cryptosporidium exposure risks, but they may have been at a reduced risk of illness. If, as indicated in a recent US study [Reference Frost13], strong serological responses are markers of protective immunity, then the low number of reported cryptosporidiosis cases in the Czech Republic could be due to high levels of protective immunity as well as limitations in disease surveillance systems.
The odds of having a strong serological response to the 15/17-kDa and the 27-kDa antigen group was less likely for the riverbank-infiltration population, suggesting that they were at much lower risk of recent infections (either symptomatic or asymptomatic) than the residents of the other sites. This unfortunately implies that the riverbank-infiltration population may also be at a higher risk of experiencing symptomatic illness should they be exposed to Cryptosporidium. However, it may be difficult to detect an increased risk of symptomatic cryptosporidiosis in this population even with increased surveillance activities. During the human dosing studies [Reference Chappell30, Reference Okhuysen31] where multiple stools were examined for each volunteer, detection of oocysts in symptomatically infected individuals was uncommon [Reference Chappell30, Reference Okhuysen31].
Large differences were observed in the mean intensity of serological responses and in the prevalence of a strong response by source/treatment of drinking water. Since responses to the 15/17-kDa antigen decline more rapidly than responses to the 27-kDa antigen, large differences, such as observed here between areas in response to the 15/17-kDa antigen, would be expected if drinking water were a primary source of exposure [Reference Muller16]. These findings add to the body of evidence indicating that differences in drinking-water sources/treatment are likely to strongly influence the level of serological responses [Reference Frost19–Reference Frost25]. The lower prevalence of serological responses to both antigens for persons residing in the area with riverbank-infiltration water agree with findings of a recent study in Hungary [Reference Frost25] where women residing in an area supplied with a riverbank-infiltration water source had a significantly lower mean serological response than women residing in areas with filtered/disinfected surface water.
Unanticipated were the results from site C, which is supplied by groundwater thought to be of very good quality. Here the proportion of seropositive subjects approximated the proportion found in cities with surface sources of water. This area is served by dozens of wells located in deeply cut valleys and drilled into sandstone and marl of the mid-turon stratum. Water circulates through a system of cretaceous sediments supplied by water accumulated in pores and replenished by the infiltration of precipitation. The recharge area is some forest but mostly agricultural land with scattered villages. During the mid-1980s, sewage disposal for many of the villages consisted of individual septic tanks. These villages now have central sewage collection and treatment. On the terrace overlooking the valleys, there are fields intensively exploited agriculturally. During the mid-1980s, there were eight large cattle and pig farms with no pasturing of the animals located within a 5-km radius from the catchment area. All waste was either composted or freely stocked in the field before working it into the soil.
Records of the quality of raw water at the water utility supplying site C were examined for the years 1984–1986. Raw water quality was checked once a month. Twelve (33·3%) of the 36 monthly raw water samples were positive for total coliforms. In one sample, 8 colony forming units (c.f.u.)/100 ml were found; in the remaining 11 samples, 1–4 c.f.u./100 ml were found. The detection frequency suggests low-levels of intermittent contamination. The concentrations of nitrates and chlorides, as well as oxygen demand and turbidity measurements were stable and satisfactory. However, the frequency of analyses was not sufficient to record all extraordinary events (e.g. penetration of surface water as a consequence of heavy rains). Moreover, the method of water treatment and the character of the geological terrain (e.g. shallow soil layer, fissured rock) provide no assurance of any reliable removal of oocysts. The water system manager reported that some wells located close to the villages had to be temporarily closed for unsuitable water quality in the 1980s. These serological findings, however, should not be surprising, since studies in the United States have reported frequent high oocyst contamination in some groundwaters [Reference Hancock, Rose and Callahan32], and a large percentage of reported waterborne outbreaks have been associated with groundwater contaminated by sewage or surface water [Reference Craun6, Reference Craun, Calderon, Craun and Pontius7].
We feel that drinking-water source and treatment are probable causes of the high seroprevalence found among the populations in sites A, B, and C, but we cannot exclude other sources of exposure that might have affected seroprevalence. Information was available on the age and occupation of the blood-sample donors, and these were not related to seroprevalence. Although the cities in site C are located near Prague, it is unlikely that persons routinely commuted to work in Prague to confound the results. Each city had sufficient industry to support the population, and the incentive to migrate or commute was minimal during the 1980s. Consumption of water from private wells was not important because practically the whole population of the four areas was connected to the public water supply. Use of bottled water during the study period was very limited because of its general unavailability on the market at that time; most persons probably drank tap water instead. Since foreign travel was also extremely limited, this was an unlikely source of exposure. It is also unlikely that there were any differences among the four areas in recreational water or day-care exposures. These and other findings in various locations [Reference Frost, Hunter, Waite and Ronchi27] suggest that the seroprevalence of Cryptosporidium responses reported here are probably related to waterborne exposure with the differences in prevalence associated with type of water source and treatment.
Although more recent seroprevalence surveys are needed to assess current infection levels and sources of exposure, these serological responses in the Czech Republic 20 years ago can help us better understand how best to reduce cryptosporidiosis. We feel that the results provide an insight into the historical levels of endemic exposure and protective immunity that may have been common in most countries at that time. The increased recognition of waterborne outbreaks of cryptosporidiosis in North America and Britain in the 1990s has resulted in changes in water treatment and operation [Reference Craun6, Reference Craun, Calderon, Craun and Pontius7]. The relatively recent emergence of cryptosporidiosis as an epidemic disease may be due, at least partially, to the success of water-treatment operators in reducing oocysts to low concentrations. By reducing low-level waterborne exposures that confer protective immunity, improved water treatment in the recent two decades may have been important in converting what had been a common infection with little apparent illness to a less common infection but a more common cause of epidemic illness. If so, it is possible that efforts to reduce or eliminate low-dose exposures to Cryptosporidium may increase rather than reduce the risk of illness because of reduced protective immunity. However, the need to eliminate periodic high-dose exposures is still an important public health activity. This can best be accomplished by improving the reliability of drinking-water treatment.
CONCLUSIONS
A number of serological surveys have shown a higher prevalence of Cryptosporidium antibodies in populations using surface water compared to populations using groundwater from confined aquifers. In this study, we found a high seroprevalence of markers of infection in an area where residents use groundwater obtained from fissured sandstone. The mean prevalence is similar to that found in areas of Hungary where groundwater is obtained from a karst aquifer and areas of the Czech Republic and Hungary where surface water is used [Reference Frost25]. A low prevalence of markers of infection was found in persons in both the Czech Republic and Hungary residing in areas where drinking water is obtained by riverbank infiltration [Reference Frost25]. The findings of these two studies suggest that riverbank infiltration is as effective in removing Cryptosporidium oocysts as conventional filtration of surface water and, depending upon the hydro-geological conditions and other water treatment provided, removal may even be more reliable. Additional seroprevalence studies should be conducted in other locations where riverbank infiltration is used to provide additional evidence of the effectiveness of this treatment in reducing waterborne exposures to Cryptosporidium.
Despite widespread exposure to oocysts in surface-derived drinking water, cryptosporidiosis outbreaks have not been reported in the Czech Republic. Protective immunity may be a partial explanation. Although the results of this study and others provide evidence to suggest that protective immunity is important for cryptosporidiosis, additional research is also needed on the role this may play in determining waterborne illness risks. In particular, the studies should evaluate the relationship between serological responses to Cryptosporidium-specific antigens and diarrhoeal or gastrointestinal illness among persons in areas served by clean groundwater, riverbank-infiltration water, and conventionally filtered surface water. Additional sources of exposure, especially foods, should also be assessed for individuals in these studies, as recent case-control studies have reported protective effects associated with eating certain raw vegetables [Reference Roy33–Reference Robertson35].
ACKNOWLEDGEMENTS
We thank the Centre of Microbiology and Epidemiology at the National Institute of Public Health in Prague for supplying the serum samples. The study was partially supported by grants for the Czech Academy of Sciences project ‘Risk of Cryptosporidium spp. Oocysts in Drinking Water’ (Reg. No. S6022006) and the Research Project (Subproject III) ‘Environmental Health Risks’.
DECLARATION OF INTEREST
None.






