Background to multiple sclerosis and its pathogenesis
Multiple sclerosis (MS) is a CNS-specific demyelinating disease, and is the most common neurological disorder that occurs in young adultsReference Ewing and Bernard1, Reference Noseworthy2. The majority of patients with MS have the relapsing-remitting form of the disease, characterised by attacks (relapses) interspersed with periods of recovery (remission). The disease is most prevalent (30–100+ cases per 100 000 people) in Western Europe, Southern Canada, Northern United States, Southern Australia and New Zealand and of low frequency (0–19 per 100,000) in Asia, Central America, Africa and Greenland (See Fig. 1). Between 2 and 3 million people Worldwide are thought to live with MS. Although the aetiology of MS remains unknown there is strong evidence for the presence of autoimmune mechanisms in the disease pathogenesisReference Martino and Hartung3, Reference Hafler4. Studies have shown that MS patients have a much higher number of neuroantigen e.g. myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG) autoreactive T-cells, which are in an increased state of activation compared with healthy controls, and which increase during exacerbationReference Hafler4–Reference Bielekova, Goodwin, Richert, Cortese, Kondo, Afshar, Gran and Eaton13.

Fig. 1 Geographical distribution of multiple sclerosis (from Adams C (1989) colour atlas of multiple sclerosis and other myelin disorders, wolfe medical publications ltd, with permission).
Cytokines from activated T cells and macrophages have been strongly implicated in the pathogenesis of MSReference Navikas and Link14. For example, the up-regulation of adhesion molecules on endothelial cells and the subsequent infiltration of activated T cells into the CNS are immunopathogenic events controlled by pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interferon-γ (IFN-γ)Reference McCarron, Wang and Racke15. Furthermore studies have shown that these cytokines exert direct myelinotoxic propertiesReference Selmaj, Raine and Farooq16, Reference Vartanian, Li and Zhao17 and prolong the disease process in experimental autoimmune encephalomyelitis (EAE), an animal model of MSReference Kuroda and Shimamoto18, Reference Issazadeh, Lorentzen and Mustafa19, Reference Ruddle, Bergman and McGrath20. TNF-α, IL-1β and IFN-γ have all been shown to be present in CNS active lesions in MS and elevated amounts of these cytokines are secreted from MS peripheral blood mononuclear cells (PBMC)Reference Cannella and Raine21–Reference Hollifield, Harbige, Pham-Dinh and Sharief25. Many studies, including our own, have also shown that an increase in these inflammatory cytokines coincides with the relapse phase of the diseaseReference Hollifield, Harbige, Pham-Dinh and Sharief25–Reference Lu, Jensen and Arnason33. Furthermore some studies have shown that transforming growth factor-beta1 (TGF-β1), a potent anti-inflammatory and immunosuppressive cytokine, is reduced during the relapse phase and increases as the patient enters remissionReference Hollifield, Harbige, Pham-Dinh and Sharief25, Reference Bertolotto, Capobianco and Malucchi34, Reference Mokhtarian, Shi and Shirazian35. In addition we have demonstrated that the balance between biologically active TGF-β1 and the pro-inflammatory TNF-α, IL-1β and IFN-γ is dysregulated during MS relapse-remissionReference Hollifield, Harbige, Pham-Dinh and Sharief25. The actual processes of axonal damage e.g. chronic inflammation, demyelination and astrogliosis in MS is complex but white matter inflammation and demyelination are considered to determine disease severity, whilst recent studies suggest that axonal damage in MS begins in the early stages of the disease and contributes to disabilityReference De Stefano, Narayanan, Francis, Arnaoutelis, Tartaglia, Antel, Matthews and Arnold36, Reference Bjartmar, Wujek and Trapp37. Furthermore some have considered metabolic disturbances in some MS patients to be behind primary oligodendrocyte damage with secondary autoimmune-demyelinationReference Lassmann, Bruck and Lucchinettii38, Reference Matute and Perez-Cerda39.
Nutritional epidemiology of multiple sclerosis
Over half a century ago Roy SwankReference Swank40 found a positive relationship between fat intake as well as annual milk production and MS in Scandinavian countries. Furthermore studies by Alter et al.Reference Alter, Yamoor and Harshe41 implicated animal fat rich in saturated fatty acids as a causal factor in MS and WolfgramReference Wolfgram42 in an analysis of World Health Organisation (WHO) annual mortality statistics found a similar geographical distribution between MS coronary heart disease and cancer of the colon. In the multivariate analysis (inclusive of socioeconomic and medical services) of 20 countries Arganoff and GoldbergReference Agranoff and Goldberg43 not only implicated meat and dairy fats in positive correlations with MS, as noted previously, but also vegetable, seed, nut and fish, foods rich in both the n-6 and n-3 polyunsaturated fatty acids, in negative correlations with MS. Several other studies also confirmed strong MS associations with dairy and other animal fatsReference Ghadirian, Jain and Ducic44–Reference Malosse, Perron, Sasco and Seigneurin46. Similar observations have also been made more recently by Esparsa et al. Reference Esparza, Sasaki and Kesteloot47 in a large study (36 countries) assessing the impact of diet on MS mortality. They found that the higher the saturated fatty acid intake the higher the MS mortality and the higher the polyunsaturated to saturated fatty acid ratio the lower the MS mortality. However the large single country study, the Nurses' Health Study in the USA failed to show any relationship between MS and fat intake in womenReference Zhang, Willet, Hernan, Olek and Ascherio48. Thus the majority of epidemiological studies indicate that foods rich in saturated fatty acids are detrimentally associated with MS, whilst polyunsaturated fatty acid rich foods are beneficially associated with MS.
Biochemical and metabolic studies of fatty acids in multiple sclerosis
There is much evidence that the n-6 fatty acids particularly linoleic (18 : 2n-6) and arachidonic acids (20 : 4n-6) are reduced in the plasma, platelets, erythrocytes, leucocytes and cerebrospinal fluid with changes in the unsaturated fatty acid composition of brain white matter in MS patients, much of this early work being undertaken at the National Hospital, Queen Square in LondonReference Baker, Thompson and Zilkha49–Reference Holman, Johnson and Kokmen60. But there are also inconsistent reportsReference Shukla and Clausen61–Reference Nightingale, Woo and Smith64and Love et al.Reference Love, Cashell, Reynolds and Callaghan65 observed that reduced linoleic acid was not specific to MS and occurred in patients with acute non-neurological illness. However many of these differences between studies are perhaps not surprising given cultural and ethnic differences, dietary variability (particularly when someone is ill), possible desaturase gene polymorphism, disease variability, serum verses cellular fatty acids and methodological differences for example total lipid fatty acids verses phospholipids fatty acid analysis.
Previously we proposed nervonic acid as a marker of CNS myelin damage in MSReference Jones, Harbige, Clifford Rose and Jones66 and found that MS patients consuming a diet rich in polyunsaturated fatty acids particularly linoleic acid had an inverse relationship between erythrocyte membrane linoleic acid and nervonic acid (24 : 1)Reference Harbige, Crawford, Jones, Preece and Forti67. A similar finding was described by Homa et al. Reference Homa, Conroy, Belin, Smith, Monro and Zilkha68, showing a decrease in erythrocyte lignoceric acid (24 : 0) in sunflower oil (rich in linoleic acid) supplemented MS patients. In an open, uncontrolled 2 year fish oil intervention study by Nordvik et al.Reference Nordvik, Myhr, Nyland and Bjerve69 in MS, they observed significant reductions in plasma total phospholipid nervonic and lignoceric acids with time and clinical improvement. Taken together the above indicate that nervonic and lignoceric acids could be useful pathogenic biomarkers of myelin damage and/or biomarkers for monitoring fatty acid treatments. We also found that the atypical erythrocyte electrophoretic response of MS patients was positively correlated with membrane linoleic acid and could be corrected by a diet rich in polyunsaturated fatty acids particularly linoleic acidReference Harbige, Crawford, Jones, Preece and Forti67. This is in agreement with Field and JoyceReference Field, Joyce and Smith71 who found an increase in erythrocyte electrophoretic response in MS patients supplemented with evening primrose oil (EPO). However, Field et al.Reference Field, Joyce and Smith71, Field and JoyceReference Field and Joyce70 interpreted their electrophoretic results, without an analysis of membrane fatty acids, as an effect of the gamma-linolenic (GLA, 18 : 3n-6) component of the oil. EPO contains about 70 % linoleic acid and 8–10 % GLA, therefore it is more likely that the effect observed by Field et al was due to the linoleic acid component of the oil rather than the GLA.
We have also investigated the metabolic relationships between the n-6 fatty acids in both healthy controls and MS PBMC total phospholipids (Figs. 2, 3 and 4). Both controls and MS patients (remission phase) demonstrate a positive correlation between linoleic acid (18 : 2n-6) and arachidonic acid (20 : 4n-6) as expected, although these n-6 fatty acids were low in a proportion of the MS patients studied (Fig. 2). Moreover, the relationship between linoleic acid (18 : 2n-6) and dihomo-γ-linolenic acid (DGLA), and also between DGLA (20 : 3n-6) and arachidonic acid (20 : 4n-6) is clearly disturbed in MS compared with healthy controls (Figs. 3 and 4). This may indicate a problem with Δ6 and Δ5 desaturation and / or a greater requirement for these n-6 fatty acids in many of the MS patients studied, about 20–30 percent of the patients showed lower than normal PBMC phospholipid DGLA and arachidonic acid. In agreement with our findings Homa et al. Reference Homa, Belin and Smith55 has also reported a similar disturbance in the relationship between linoleic acid and arachidonic acid in MS erythrocyte membrane lipids compared to healthy controls. Furthermore when we compared MS and healthy control PBMC total phospholipid 20 : 2n-6 we found a significant 2 fold higher 20 : 2n-6 in MS patients in remission compared to healthy controls and a significant 4 fold higher 20 : 2n-6 in the relapse phase of the disease. It appears that in MS there is a very active elongation of 18 : 2n-6 to 20 : 2n-6 in PBMCs and that this is even higher in the relapse phase (accounting for the low 18 : 2n-6) indicating a disturbance in the normal metabolism or a higher requirement for DGLA and arachidonic acid (20 : 2n-6 maybe further Δ8 desaturated to DGLA?) by these n-6 fatty acid (20 : 4n-6) rich cellsReference Harbige72, or both. This may also be reflective of the demand of cells and myelin in the brain which are also n-6 fatty acid-rich (20 : 4n-6 and 22 : 4n-6), significantly Stanley Rapoport of the NIH has shown that the human brain requires 4 times the amount of arachidonic acid (20 : 4n-6) than docosahexaenoic acid (22 : 6n-3) on a daily bases (ISSFAL 2006). In relation to 20 : 2n-6, although not discussed by the authors, the Nordvik et al.Reference Nordvik, Myhr, Nyland and Bjerve69 study demonstrated a reduction with time of 20 : 2n-6 running parallel with clinical improvement, a similar finding to the nervonic and lignoceric acids mentioned earlier. Therefore 20 : 2n-6 may also be a useful marker of disease progression and/or monitoring fatty acid treatments in MS.
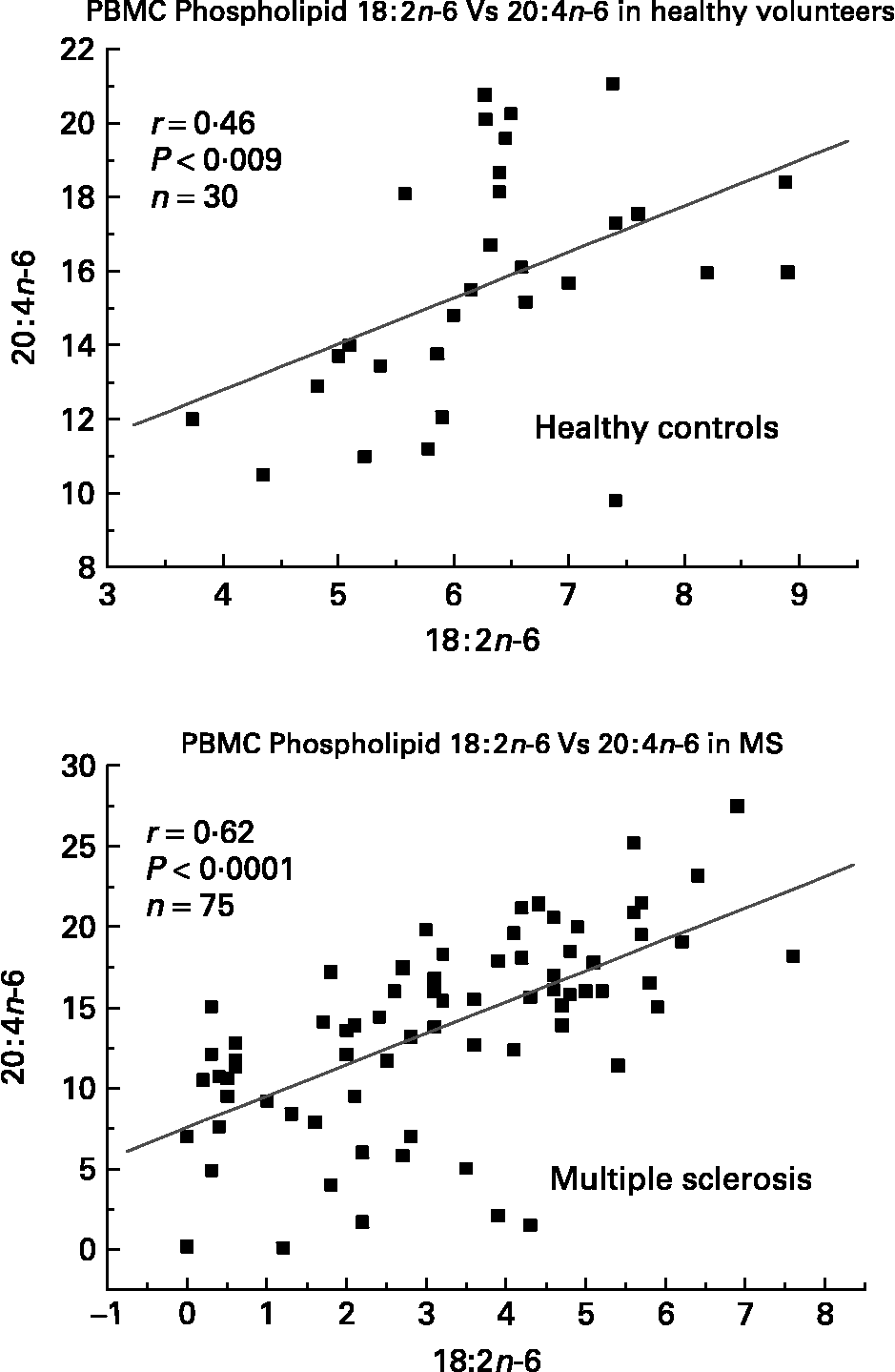
Fig. 2 Relationship between linoleic acid (18 : 2n-6) and arachidonic acid (20 : 4n-6) in peripheral blood mononuclear cell total phospholipids of healthy controls and multiple sclerosis.
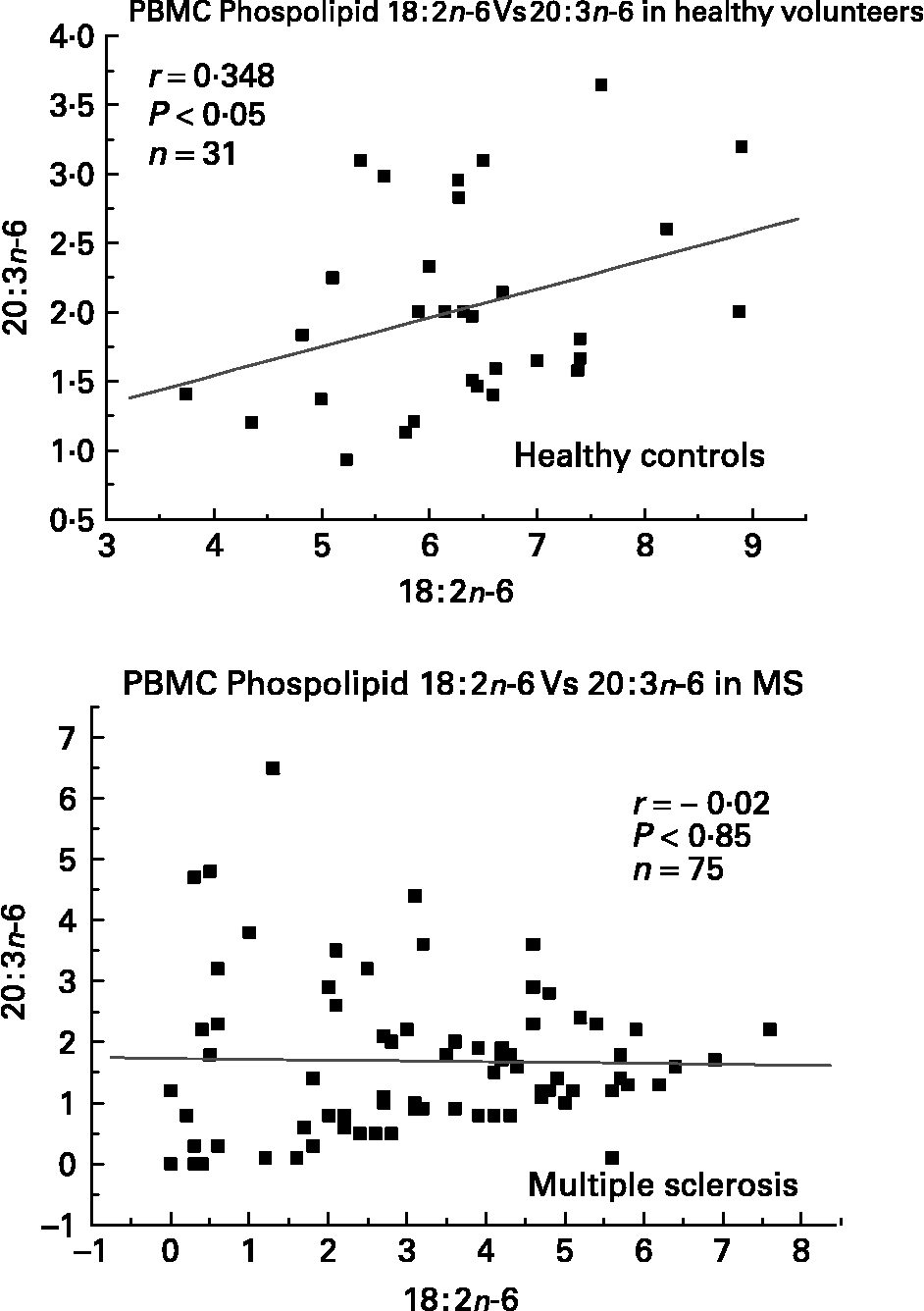
Fig. 3 Relationship between linoleic acid (18 : 2n-6) and dihomo-γ-linolenic acid (20 : 3n-6) in peripheral blood mononuclear cell total phospholipids of healthy controls and multiple sclerosis.
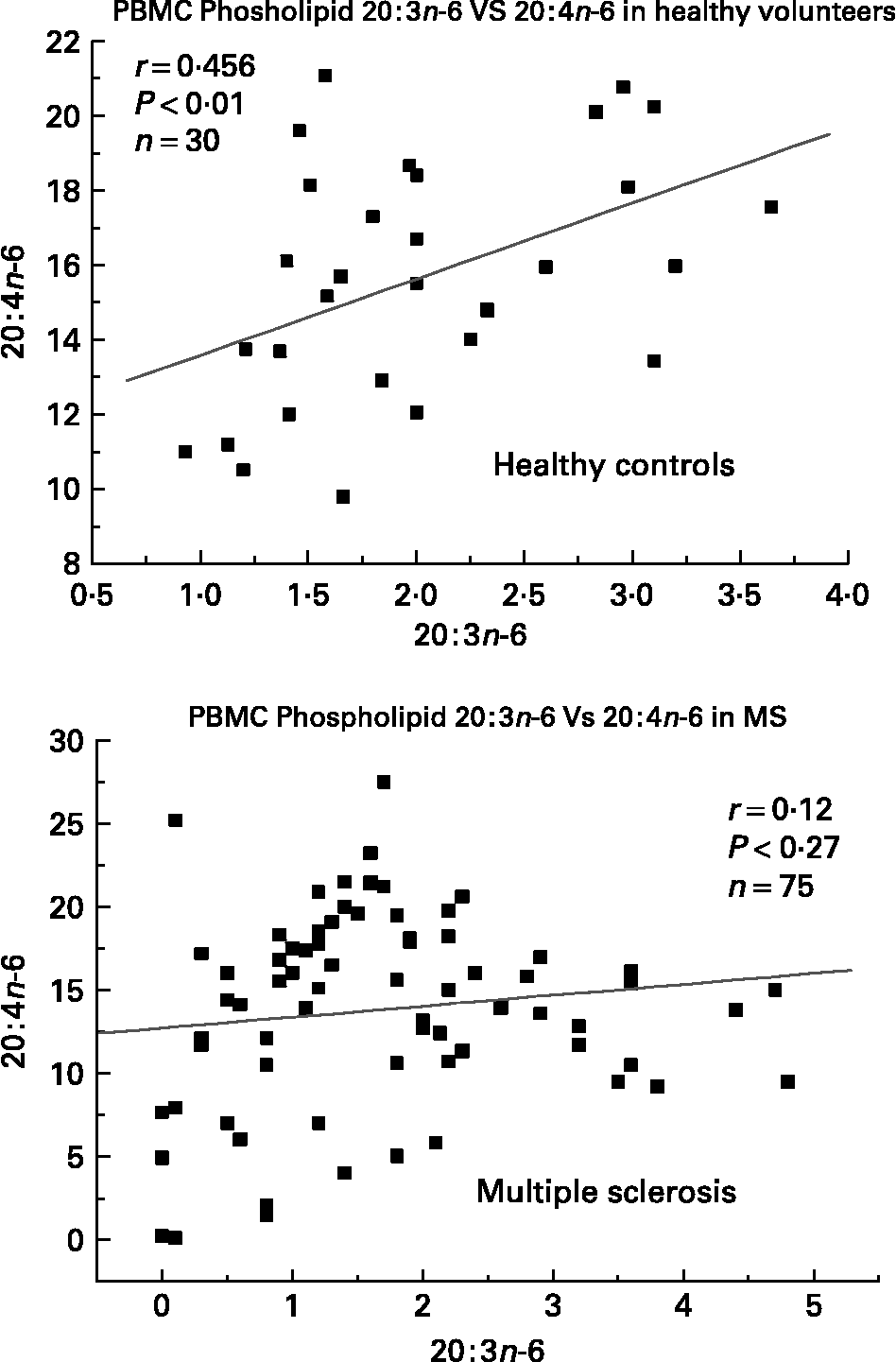
Fig. 4 Relationship between dihomo-γ-linolenic acid (20 : 3n-6) and arachidonic acid (20 : 4n-6) in peripheral blood mononuclear cell total phospholipids of healthy controls and multiple sclerosis.
Fatty acids and animal models of multiple sclerosis
Experimental autoimmune encephalomyelitis (EAE) is an experimentally induced CD4+T cell mediated autoimmune-inflammatory and deyelinating disease in rodents often used as an animal model of MS. Studies in guinea pig and rat EAE treated with linoleic acid alone or a high linoleic and low γ-linolenic (GLA) acid rich oil (ratio 7 : 1) respectively, have shown partial suppression of the incidence and severity of EAEReference Meade, Mertin, Sheena and Hunt73, Reference Mertin and Stackpoole74. In a series of experiments we demonstrated important disease modifying effects of linoleic acid-rich oil (containing no GLA) and GLA-rich oil on clinical and histopathological manifestations of EAE. Depending on dose GLA was completely protective in EAE, whereas linoleic acid had a dose dependent action on the clinical severity of EAE, although not abolishing itReference Harbige, Yeatman, Amor and Crawford75, Reference Harbige, Layward and Morris-Downes76. Natural recovery in EAE is mediated by expansion of suppressor lymphoid cellsReference Adda, Beraud and Depieds77 some of which have been characterised as TGF-β producing CD4+T cells by Karpus and SwanborgReference Karpus and Swanborg78. Furthermore administration of TGF-β protects in acute and relapsing EAEReference Rack, Sriram, Calrlini, Cannella, Raine and McFarlin79, Reference Santambrogio, Hochwald, Saxena, Leu, Martz, Carlino, Ruddle, Palladino, Gold and Thorbecke80 and prostaglandin inhibitors such as indomethacin augment EAEReference Ovadia and Paterson81. In-addition during the natural recovery phase from EAE TGF-β secreting T cells can inhibit EAE effector cells and TGF-β is expressed in the CNSReference Karpus and Swanborg78, Reference Liblau, Singer and McDevitt82, Reference Khoury, Hancock and Weiner83. Consistent with these findings the protective effect of GLA-rich oil in EAE is linked to increased T cell TGF-β transcription and increased production of PGE2Reference Harbige, Layward and Morris-Downes76.
Clinical trials and intervention studies in multiple sclerosis with fatty acids
Clinical trials to test the efficacy of linoleic acid-rich sunflower oil in MS patients by Miller et al. Reference Bates, Fawcett, Shaw and Weightman84 and Bates et al. Reference Millar, Zilkha, Langman and Payling-Wright85 over 2 years showed a reduction in the relapse rate and severity of the disease relapse, but Paty et al. Reference Paty, Cousin, Read and Adlakha86 found no such effect. Nevertheless, Dworkin et al. Reference Dworkin, Bates and Millar87 in a statistical revaluation of the combined data of all three trials revealed significantly reduced relapse rate and severity, and in mildly affected a decrease in the long term progression of the disease. The Millar et al. Reference Bates, Fawcett, Shaw and Weightman84 study based in two centres London and Belfast is particularly interesting as they observed that “the severity of the relapses, differed markedly between the treated and the control groups at both centres” relapses being twice as severe in the control group. Compared with current β-interferon treatment of MS the efficacy of linoleic acid-rich sunflower oil in the Miller et al study is quite remarkable. Fish oil rich in long chain n-3 fatty acids has also been studied in MSReference Bates, Cartlidge and French88, no significant differences between fish oil treated and untreated MS patients was observed, there was, however, a trend for less deterioration in the fish oil treated group. In a 2 year open intervention study MS patients given fish oil and advised to lower their saturated fat intake had significant reductions in the relapse rate and disability progression as measured by the Expanded Disability Status Scale (EDSS)Reference Nordvik, Myhr, Nyland and Bjerve69 which quantifies disability in MS in eight functional systems (pyramidal, cerebellar, brainstem, sensory, bowel and bladder, visual, cerebral, other). It also appears based on open studies by us and by Roy Swank that long term low saturated fat diets containing both n-6 and n-3 fatty acids improve the course of the diseaseReference Swank89–Reference Harbige, Jones, Jenkins, Fitzgerald, Forti and Budowski93.
Based on our MS fatty acid metabolic data and experimental animal model work, described above, we undertook a randomised double-blind placebo controlled trial to determine the effects of supplementation with a selected GLA (18 : 3n-6)-rich borage oil. This oil, BGC20-884 was high in sn-2 GLA, low in monoenes and contained only natural levels of vitamin E. This study evaluated two doses of BGC20-884 (low dose - 5 gram and high dose - 14 gram per day) and a placebo control (polyethylene glycol 400) on the clinical course and PBMC cytokine and membrane fatty acid profiles of 36 patients with active MS over 18 monthsReference Harbige, Hollifield, Pinto, Xiang, Leach and Sharief94. Patients were diagnosed and assessed using international criteria for MS. Relapse rate and EDSS (Expanded Disability Status Scale) were assessed every three months and blood taken and PBMCs isolated for cytokine studies and membrane fatty acids. High dose BGC20-884 treatment markedly and significantly reduced the relapse rate (Fig. 5) and disability progression as measured by EDSS (Fig. 6) compared with the placebo control and low dose BGC20-884 treatment. In patients where we had follow up samples available PBMC cytokine changes were found to run parallel with the clinical findings e.g. the placebo control group showed significant decreases in the TGF-β/TNF-α and TGF-β/IL-1β ratios and associated loss of n-6 fatty acids particularly linoleic (18 : 2n-6) and arachidonic acid (20 : 4n-6) over time. Consistent with our findings Navarro and SeguraReference Navarro and Segura59 also found significant loss of linoleic and arachidonic acids over time in MS erythrocyte phospholipids. In contrast high dose BGC20-884 treatment showed no changes in TGF-β/TNF-α and TGF-β/IL-1β ratios and no changes in membrane n-6 fatty acids compared with the placebo group. We also found positive correlations between PBMC phospholipid arachidonic acid composition and TGF-β1 production (r = 0·26, P < 0·02, n = 73) and DGLA and TGF-β1 production (r = 0·36, P < 0·001, n = 74) ex vivo when all samples were included in the analysis. The EDSS improvement in the high dose group also suggests there maybe a beneficial effect on neuronal lipids and neural function in MS. The study thus further supports our hypothesis of dysregulation of fatty acid metabolism and cytokines in MSReference Hollifield, Harbige, Pham-Dinh and Sharief25, Reference Harbige, Layward and Morris-Downes76.
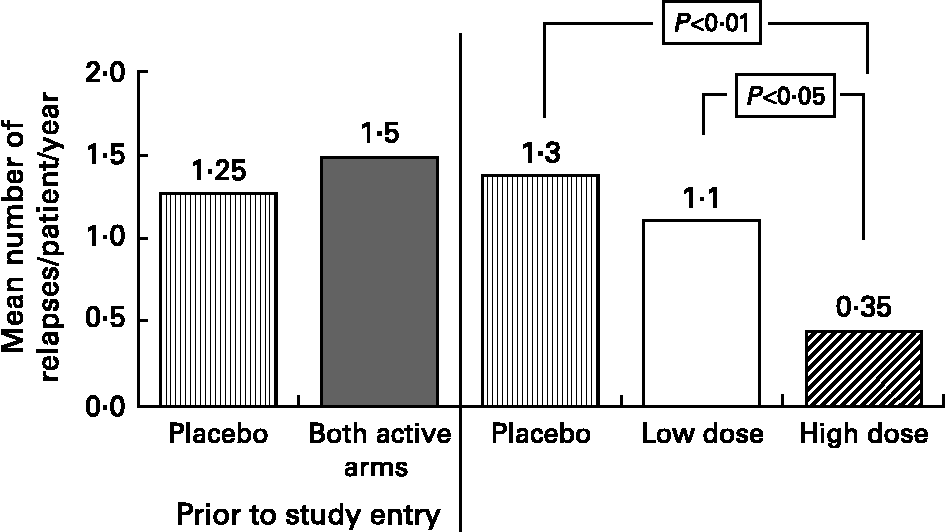
Fig. 5 Mean annualised relapse rate per patient for multiple sclerosis patients receiving high (n = 11) and low dose (n = 7) GLA-rich oil or placebo control (n = 10) over 18 months.
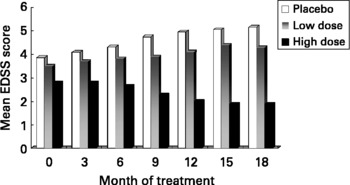
Fig. 6 Disability progression as measured by the EDSS (Expanded Disability Status Scale) in multiple sclerosis patients receiving high (n = 11) and low dose (n = 7) GLA-rich oil or placebo control (n = 10) over 18 months.
To summarise and extend this section on clinical trials there is evidence to show a beneficial effect of n-6 and possibly n-3 fatty acids in MS. The mechanisms by which the n-3 or the n-6 fatty acids influence the immune-inflammatory response in MS are however likely to be differentReference Harbige72. Both the n-6 (arachidonic acid) and n-3 (docosahexaenoic acid) fatty acids are important for neural structure and functionReference Neuringer, Anderson and Conner95–Reference Conklin, Gianaros and Brown101 and this aspect may explain studies where improvements in EDSS have also been reported. Furthermore requirements for essential polyunsaturated fatty acids increase as a function of the amount of saturated fat in the dietReference Holman102 and we have recently found significant positive correlations between dietary total saturated and total monounsaturated fatty acids and delta-6 and delta-5 desaturase gene expression in human PBMCReference Xiang, Rahman, Ai, Li and Harbige103. The level of dietary saturated and monounsaturated fatty acids should not therefore be ignored and may be important factors in some of the trials discussed above and relate to the MS epidemiological correlations, mentioned earlier in this overview, in relation to an increased requirement for polyunsaturated fatty acids in MS.
Conclusions and perspectives
Taken overall the epidemiological, biochemical, experimental animal model and clinical trial data described in this overview show that polyunsaturated fatty acids, particularly the n-6 fatty acids, do have a role in the pathogenesis and treatment of multiple sclerosis. We have demonstrated dysregulation of n-6 fatty acid metabolism and cytokines in MS and have been able to show in a small “proof of concept” clinical trial a marked therapeutic benefit. Thus we suggest that dysregulation of n-6 fatty acid metabolism and cytokines is one mechanism that is important in disease progression, which is modifiable by specific supplementation. Thus metabolic disturbance of the production of the long chain n-6 fatty acids DGLA and AA affects the physiological integrity of immune cells, in that they have a limited ability to produce TGF-β, under relapse conditions, which is important for the regulation of pro-inflammatory cytokine production e.g. TNF-α, IL-1β, IFN-γ as well as other cellular biological functions. It is also known that TGF-β and fatty acids such as arachidonic acid are important in the growth and differentiation of oligodendrocytes and in myelinationReference Sinclair and Crawford104–Reference Van Meeteren, Baron, Beermann, Dijkstra and van Tol108 which would therefore be of importance in the stimulation, growth and recovery of these cells in MS. These findings provide a link between, dietary, metabolic, immunological and neurobiological aspects of MS and therefore for the first time we can begin to make sense of the wealth of apparently unconnected aspects of MS, particularly in relation to dietary fats. More basic research is still required such as characterisation of possible desaturase gene polymorphisms, lymphocyte desaturase gene expression and analysis of specific lymphocyte phospholipid classes and their fatty acid composition in relation to cytokine and chemokine gene expression and production. This should be undertaken in well defined MS patient groups e.g. active MS and primary and secondary chronic progressive forms of the disease and over an extended period of time. Furthermore large well controlled clinical trials with different doses of well characterised and safe fatty acid formulations as well as manipulation of dietary saturated fatty acids could be undertaken. Clinical trials should include MRI, MR spectroscopy and analysis of lesion burden and cortical gray matter density in order to investigate any possible effects of polyunsaturated fatty acids on myelination, neuronal, dentritic, glial and neurite packing densities. In addition biochemical monitoring of peripheral blood cell membrane phospholipid fatty acids, particularly lymphocytes, should be undertaken as well as immunological studies such as T-cell and macrophage pro- and anti-inflammatory cytokine gene expression and production, T regulatory cells and anti-myelin antibodies. In this way a more complete picture will emerge of the clinical and therapeutic significance and the metabolic, immunological and neurological bases to the role of polyunsaturated fatty acids in the pathogenesis and treatment of MS.
Conflict of interest statement
BGC20-884 and related intellectual property are patented by BTG International Ltd with LSH and MKS as named inventors. LH and MKS co-wrote the manuscript. At the time of the trial there were no conflicts of interests. Subsequently to the trial findings BGC20-884 and related intellectual property is now the subject of patents held by BTG International Ltd with LSH and MKS as named inventors. LH wrote the text and MKS was the lead trial neurologist.










