The efficient utilization of dietary protein is a key issue of ruminant nutrition. In the rumen, 70–80 % of the protein is degraded via peptides and amino acids to ammonium and SCFA including branched chain fatty acids (Barry & McNabb, Reference Barry and McNabb1999; National Research Council, 2001). On protein-rich diets used in intensive production systems, proteolysis by ruminal micro-organisms becomes a wasteful process, if dietary protein is broken down in excess of the requirements for maximal microbial growth and degradation of the feed (Leng & Nolan, Reference Leng and Nolan1984). The amount of protein available to the ruminant depends on the combination of microbial protein reaching the small intestine and the amount of dietary protein that escapes microbial degradation (by-pass protein). To sustain high productivities, high-yielding dairy cows need large quantities of by-pass protein that can be hydrolysed and absorbed post-ruminally (Santos et al. Reference Santos, Santos, Theurer and Huber1998). Thus, the proportion of by-pass protein constitutes one of the major factors of feed N value (Michelet-Doreau & Ould-Bah, Reference Michelet-Doreau and Ould-Bah1992).
Ionophores have often been used as feed additives to increase feed conversion efficiency in ruminants. Part of the mode of action of ionophores involves improved protein utilization, which results from effects on ruminal protein metabolism. The ionophores, monensin, salinomycin and tetronasin, have protein-sparing effects that are mediated by decreased breakdown of protein, peptides and amino acids (Van Nevel & Demeyer, Reference Van Nevel and Demeyer1977; Newbold et al. Reference Newbold, Wallace and McKain1990; Yang & Russell, Reference Yang and Russell1993; McAllister et al. Reference McAllister, Bae, Yanke, Cheng and Muir1994). The ban of growth-promoting antimicrobials by the European Union in 2006 means that additives such as ionophores can no longer be used in Europe, which highlights the need for alternative ways to promote efficient N utilization in ruminants. Plants or plant-derived products offer a promising alternative (Wallace, Reference Wallace2004).
Studies of plants and phytochemicals that inhibit ruminal proteolysis have generally involved tannin-containing extracts or tannin-rich plants (McMahon et al. Reference McMahon, McAllister, Berg, Majak, Acharya, Popp, Coulman, Wang and Cheng2000; Hervás et al. Reference Hervás, Ramos, Giráldez and Mantecon2004; Martínez et al. Reference Martínez, Moyano, Barroso and Alarcón2004). By forming complexes with proteins, tannins decrease the availability of soluble protein to proteases and thus decrease proteolysis (Tanner et al. Reference Tanner, Moore and Larkin1994). In vivo studies have shown that tannins from various sources led to decreased ruminal NH3 concentrations and an enhanced non-NH3-N flow to the duodenum in sheep (Hervás et al. Reference Hervás, Frutos, Serrano, Mantecon and Giráldez2000; Śliwiński et al. Reference Śliwiński, Kreuzer, Wettstein and Machmüller2002). However, tannins cause adverse effects such as decreased feed intake (Silanikove et al. Reference Silanikove, Perevolotsky and Provenza2001), a decline in fibre digestibility (McAllister et al. Reference McAllister, Bae, Yanke, Cheng and Muir1994; Barry & McNabb, Reference Barry and McNabb1999; Hervás et al. Reference Hervás, Frutos, Giráldez, Mantecon and Alvarez Del Pino2003) and a decrease in post-ruminal degradation of protein (Hervás et al. Reference Hervás, Frutos, Serrano, Mantecon and Giráldez2000). Saponins and saponin-containing plants also improve protein flow from the rumen, but this appears to be mediated mainly by suppressing ciliate protozoa (Wina et al. Reference Wina, Muetzel and Becker2005).
The EC project, ’Rumen-up’ (QLK5-CT-2001-00 992, http://www.rowett.ac.uk/rumen_up/), was commissioned in order to explore plant-based alternatives to antimicrobial growth promoters in ruminants. One of its aims was to find plants or their extracts that decrease ruminal proteolysis. Here we report the findings of the screening programme. The results demonstrate that, although most samples that inhibited proteolysis did so via their tannin content, Knautia arvensis (field scabious) inhibited proteolysis by a different mechanism, which was more readily detected in vitro in growing batch cultures rather than by direct measurement of the proteolytic activity of digesta.
Materials and methods
Plant samples
Plant samples were derived from the ’Rumen-Up’ collection. The collection comprised 450 samples of plant parts, the great majority consisting predominantly of foliage, and fifty essential oil compounds. The species and compounds comprising the collection can be found online (http://www.rowett.ac.uk/rumen_up/). After collection, plants were freeze-dried, ground to pass through a 1 mm sieve and stored in glass jars in the dark.
Animals, diets and preparation of ruminal digesta
Three ruminally cannulated adult sheep received a maintenance diet comprising grass hay, rolled barley, cane molasses, fish meal and minerals and vitamins (Lamscov Intensive Lamb 317; Norvite, Insch, Aberdeenshire, UK) at concentrations of 500, 299·5, 100, 91 and 9·5 g/kg DM, respectively, fed in equal meals of 500 g at 08.00 and 16.00 hours. Samples of ruminal fluid were removed 2 h after the morning feeding and strained through two layers of muslin cloth before use in experimental measurements.
Five ruminally cannulated lactating Holstein cows received a total mixed ration fed ad libitum (18–19 kg) in two equal meals at 08.00 and 16.00 hours. The ration contained legume seeds (Lupinus angustifolius), maize silage, hay, maize kernel, grass silage, wheat and minerals at concentrations of 300, 274, 140, 132, 83, 44, 27 g/kg DM. Contents of organic matter, crude protein, crude fibre and metabolizable energy were 926, 163, 184 g and 11·4 MJ per kg DM. Ruminal digesta was collected prior to morning feeding. Liquid digesta was prepared by manually pressing out liquid from the feed mat into preheated thermos flasks. The fluid was filtered through a 100 μm nylon net and diluted 1:10 with pre-warmed reduced buffer medium (13·5 mm-NH4(CO3)2, 86·5 mm-Na(CO3)2, 5·5 mm-Na2HPO3, 9·5 mm-KH2PO3, 0·5 mm-MgSO4·7 × H2O, 0·020 % microminerals (0·45 m-CaCl2, 0·25 m-MnCl4, 0·02 m-CoCl6, 0·15 m-Fe(Cl)3), 6 % reducing solution (0·118 m-cysteine HCl, 0·04 % 1 m-NaOH, 0·026 N Na2S), 0·001 % resazurin).
Screening for effects on proteolytic activity
The effects of all 500 samples on rumen microbial proteolytic activity were investigated using casein (Sigma Chemical Co, Poole, Dorset, UK) reductively methylated with [3H] or [14C]formaldehyde (Wallace, Reference Wallace1983) as substrate and ruminal digesta obtained from the sheep. The assay contained 1·0 ml strained ruminal fluid, to which was added 3·0 ml anaerobic 50 mm-potassium phosphate buffer, pH 7·0, containing 4 mg 14C-labelled casein/ml. Ground sample was added to the incubation mixture to give a final concentration of 1 g/l. Essential oils were added in ethanolic solution, to final concentrations of 100 ppm. After 30 min incubation at 39°C, the reaction was stopped by the addition of 1 ml 25 % TCA. Samples were chilled at 4°C and then centrifuged at 13 000 g for 5 min. Incubations were carried out in duplicate. Acid-soluble 14C in the supernatant fluid was measured by liquid-scintillation spectrometry. The results were analysed by ANOVA using Genstat 6 software (VSN International Ltd., Hemel Hempstead, UK).
Protein degradation studies using batch culture
A batch culture method was adopted that was based on the method developed by Mauricio et al. (Reference Mauricio, Mould, Dhanoa, Owen, Channa and Theodorou1999). Incubations were run in 125 ml serum flasks at 39°C under CO2. Of the buffered bovine digesta 75 ml was added to pre-warmed flasks containing a substrate mix consisting of 450 mg maize silage and 225 mg barley grain ground to 1 mm. Bovine serum albumin (BSA; A9647, Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany; protein content ≥ 96 %) and soyabean flour (S9633; protein content approximately 52 %) were added to final concentrations of 0·13 and 2·0 g/l, respectively. Crude nutrients (Association of Official Analytical Chemists, 1990) and fibre contents (Van Soest et al. Reference Van Soest, Robertson and Lewis1991) of the single substrates and of the final mixture are given in Table 1. Plants were added at an inclusion level of 18 % (w/w) of total substrate, replacing the corresponding amount of maize silage. To estimate the impact of tannins present in plant samples, parallel incubations were performed in the presence of 6 g/l polyethylene glycol (PEG) (PEG-6000; Merck, Darmstadt, Germany). Three flasks containing buffered rumen fluid served as blanks to correct for gas release and fermentation products originating from the inoculum. Three flasks containing buffered rumen fluid and basic substrate plus respective protein supplement served as controls. Three parallels were inoculated per treatment with one replicate being reserved for gas readings up to 12 h of incubation. Aliquots (1 ml) were withdrawn from the remaining two replicates periodically under vigorous stirring and centrifuged immediately (30 000 g, 10 min, 4°C). The pellet and 50 μl supernatant were used for analysis of the protein fractions. To the remaining supernatant (630 μl), 70 μl formic acid containing an internal standard (1 % methylbutryric acid) was added and proteins were precipitated overnight at 4°C. Samples were centrifuged (30 000 g, 10 min, 4°C) and the supernatant was collected for analysis of SCFA and NH3.
Table 1 Crude nutrient and fibre contents of major substrate components and of the final mixture used in the batch-culture system*†
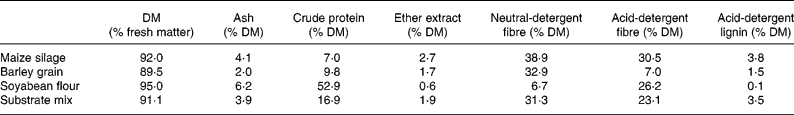
* Final substrate mix contained (mg): maize silage 450; barley grain 225; soyabean flour 150; bovine serum albumin 10.
† For details of diets and procedures, see Materials and methods.
Incubations with monensin (Sigma-M5273) served as standard in all experiments. A stock solution in ethanol was prepared freshly before incubations and 11·25 μl was dosed into each flask immediately after filling. The resulting ethanol concentration was shown to have no measurable effects on fermentation. The final concentration (3 μm) chosen lay at the lower end of concentrations of 3–9 μm commonly used in in vivo studies (Callaway et al. Reference Callaway, Russell and Carneiro De Melo1997; Ramanzin et al. Reference Ramanzin, Bailoni, Schiavon and Bittante1997; Phipps et al. Reference Phipps, Wilkinson, Jonker, Tarrant, Jones and Hodge2000).
Biochemical analyses
Concentrations of SCFA were determined by GC using a stainless steel column packed with GP 10 % SP 1000 1 % H3PO4, Chromosob WAW (Supelco Inc., Bellafonte, PA, USA) (Hoeltershinken et al. Reference Hoeltershinken, Plitt, Tammen, Hoffmann and Scholz1997). NH3 concentration was measured by the phenol-hypochlorite method (Koroleff, Reference Koroleff and Grasshoff1976) adapted to microtitre plate dimensions. Soluble protein was determined by dot blot directly from the supernatant as described in Hoffmann et al. (Reference Hoffmann, Muetzel and Becker2002). Pellets were solubilized in denaturing buffer (Laemmli, Reference Laemmli1970) before blotting to determine insoluble protein. Total protein was calculated from the sum of the two protein fractions. Individual protein bands were quantified after separation by SDS-PAGE (Laemmli, Reference Laemmli1970). Samples of 6 μl (0·5–2·0 g/l protein) were loaded on discontinuous polyacrylamide gels (Hoefer system ‘mighty small II’) with 15 % acrylamide in the separating gel. Gels were run for 60 min (10 min at 25 mA, 50 min at 40 mA), fixed in 10 % sulfosalicylic acid for 30 min and stained with Coomassie Brilliant Blue (Neuhoff et al. Reference Neuhoff, Stamm and Eibel1985). Broad range markers (1610317; Bio-Rad Laboratories GmbH, Munich, Germany) were used as molecular weight standards. Gels were documented with a digital camera system (DIANA 1·6; Raytest GmbH, Straubenhardt, Germany) and representative protein bands were quantified by image analysis (AIDA 2·31; Raytest).
For tannin analyses, freeze-dried plant samples were ground to a fine powder and tannins were extracted in 50 % (v/v) aqueous methanol (Makkar et al. Reference Makkar, Dawra and Singh1988a). Total phenols and total precipitable phenolics (’total tannins’) were determined by the ferric chloride assay using the entire extract, or after tannin-protein precipitation with BSA solution (2 g/l), respectively (Hagerman & Butler, Reference Hagerman and Butler1978).
Statistical analyses and calculation of protein degradation rates
Results obtained from batch incubations were analysed for effects of additives, of plant samples as well as of monensin, on fermentation parameters using a general linear model (SAS V9·1; SAS Institute Inc, Cary, NC, USA). Degradation of soluble protein followed a linear regression (mean coefficient r 2 0·918, n 20). Thus, protein degradation rates (k d) were calculated from protein concentrations (B) at individual sampling times (t) or from regression curves over entire incubation according to the formula:
Fraction B was set to 100 % assuming that soluble protein is fully degradable.
Results
Screening of 500 samples for inhibition of proteolysis
Plant samples were derived from the ’Rumen-Up’ collection, which comprised 450 samples of plant parts and fifty essential oil compounds. The entire collection was screened for effects on general fermentation parameters (not shown) to omit samples negatively influencing digestibility.
The samples were screened for their effects on proteolysis at a concentration of 1 g/l using a 30-min assay based on the digestion of radiolabelled casein. Subsequently, twenty-one samples testing positive initially for inhibitory activity were tested further in a 12-h batch culture system containing a 10 % inoculum. A short list of promising samples was drawn up to be investigated in more detail. Four samples, Helianthemum canum, Erica arborea, Arbutus unedo and Arctostaphylos uva-ursi, were chosen because of their inhibition of 14C-labelled casein degradation. Four additional samples, Daucus carota, Clematis vitalba, Knautia arvensis and Peltiphyllum peltatum, were chosen because they caused decreased NH3 and/or branched SCFA concentrations in the batch culture. The origins, common names and further details of the selected samples are given in Table 2.
Table 2 Description of plant samples selected for detailed screening*

* For details of procedures, see Materials and methods.
NS, not specified.
Influence of selected samples on digestion of protein and fermentation in vitro
The selected samples were added to the batch culture system at an inclusion level of 18 % (w/w) total substrate, replacing the corresponding amount of maize silage. Incubations were run for 12 h; since previous runs had shown that maximal fermentation rate (determined by maximal gas production rate) was reached after 4–6 h (in 82 % of seventeen independent incubations, mean 4·6 h, earliest after 3 h, latest after 8 h) and declined afterwards. After disappearance of soluble substrate protein, soluble protein concentration rose again after 12–15 h, most probably due to autolysis of micro-organisms.
From repeated independent incubations, the effects of the plant additives and of monensin were assessed as a percentage of values obtained in control flasks at the respective sampling times, with a 3 μm-monensin treatment included as a positive, i.e. inhibitory, reference material (Table 3). Neither any of the eight plant samples nor 3 μm-monensin showed a significant impact on SCFA production, indicating that carbohydrate fermentation was not negatively influenced. Five samples proved to have significant effects on protein degradation. P. peltatum, H. canum, A. unedo and A. uva-ursi strongly decreased the concentration of NH3 and branched SCFA. The effects of P. peltatum and H. canum on branched SCFA production were particularly marked, causing >70 % inhibition. With P. peltatum, H. canum, A. unedo and A. uva-ursi, insoluble protein concentration after 12 h of incubation tended to be higher than in the control, with the effect reaching significance (P < 0·05) for P. peltatum, H. canum and A. uva-ursi. K. arvensis addition led to a decreased concentration of branched SCFA but not NH3 at 12 h. The mechanism whereby K. arvensis caused the inhibition was apparently different to the other four samples, in that K. arvensis increased the final concentration of soluble, but not insoluble, protein after 12 h incubation, whereas the others caused a small decrease in soluble protein concentration and an increase in insoluble protein. The others also caused 8–18 % decreases in gas production, while K. arvensis did not inhibit gas production. Only minor effects on the major SCFA were observed with the plant samples. The effects of K. arvensis resembled those of monensin in decreasing soluble protein degradation and branched SCFA production, but differed in that K. arvensis did not decrease NH3 formation, the C2:C3 ratio or gas production. Degradation rates of soluble protein in controls, monensin and K. arvensis flasks were significantly different (P = 0·0001) amounting to 0·085, 0·039 and 0·068 per h after 9 h, and 0·071, 0·061 and 0·040 per h when calculated from the slope of protein kinetics.
Table 3 Relative values of fermentation parameters in 12-h batch-culture incubations with eight plant materials and 3 μm-monensin‡

All values are concentrations relative to concentrations in control incubations 100, except gas production, which is expressed as ml/g substrate with the control value 100. Values are means of four independent runs with different rumen inocula, except for E. arborea, H. canum and A. uva-ursi (three independent runs). Mean values were significantly different from control; *P < 0·05; **P < 0·01; ***P < 0·001; †P < 0·09.
‡ For details of procedures, see Materials and methods.
Assessment of mechanisms of inhibition
The effects of P. peltatum, H. canum, A. unedo and A. uva-ursi were similar, characterized by decreases in soluble protein concentration and larger increases in insoluble protein remaining, with the end products of proteolysis being decreased. Protein precipitation by P. peltatum, and the corresponding absence of precipitation by K. arvensis, was illustrated by SDS-PAGE (Fig. 1). When K. arvensis was added, the substrate protein remained soluble and was degraded over time, as demonstrated by the fading of the protein bands after 6 and 12 h, in particular of the two major soya bands (Fig. 1(C)). When P. peltatum was added, apart from a faint BSA band substrate protein was not detected in the soluble fraction, but appeared in the pellet fraction and the decrease of band intensities over time was less pronounced. After 12 h incubation, soya protein band 2 was still clearly visible (Fig. 1(D)). The percentage of protein precipitated was highest at the beginning of the incubation, when soluble protein concentration in the control was highest.

Fig. 1 Kinetics of soluble protein concentration in batch-culture incubations with Knautia arvensis and Peltiphyllum peltatum with and without the addition of polyethylene glycol (PEG). Protein degradation was determined by dot blot analysis and PAGE protein banding patterns of soluble and insoluble protein. (A) Kinetics of control (–◆–); K. arvensis (–□–); K. arvensis + PEG (–○–); 3 μm-monensin (–▲–). (B) Kinetics of control (–◆–); P. peltatum (–□–); P. peltatum + PEG (–○–). (C) Banding patterns of supernatant and pellet subsamples after 1, 6 and 12 h incubation with K. arvensis. (D) Banding patterns of supernatant and pellet subsamples after 1, 6 and 12 h incubation with P. peltatum. BSA, bovine serum albumin. For details of procedures, see Results.
The influence of samples on proteolytic activity in short incubations of ruminal digesta with 14C-labelled casein indicated that there was no immediate effect of K. arvensis (Table 4). P. peltatum, H. canum, E. arborea, A. unedo and A. uva-ursi gave inhibition that ranged from 9–28 %. Precipitation of soluble protein in the batch culture system was evident after 1 h with P. peltatum, H. canum, A. unedo, A. uva-ursi and E. arborea, ranging from 18–61 % of control values (Table 4). Chemical analysis revealed high concentrations of phenolic compounds in five plants, namely, P. peltatum, H. canum, E. arborea, A. unedo and A. uva-ursi (Table 4). Concentrations of tannins followed a similar pattern. K. arvensis had a low content of total phenolic compounds and no detectable tannins. The ability of the plant samples to precipitate BSA corresponded to their tannin content with a correlation coefficient r 2 0·60. The low correlation was due to E. arborea, which exhibits a high BSA precipitating activity; when E. arborea was excluded from the correlation, the coefficient between BSA precipitation and total tannins was r 2 0·98. Total tannins were negatively correlated, with the percentage of mixed substrate protein remaining soluble after 1 h batch-culture incubation, r 2 0·84, and for the proteolytic activity measured by the 14C-method, r 2 0·80. Further, total tannin content was negatively correlated with concentrations of end products after 12 h in batch cultures, r 2 0·82 with branched SCFA and r 2 0·86 with NH3 concentration. Protein precipitation by P. peltatum, H. canum, A. uva-ursi, A. unedo and E. arborea was partly reversed by the addition of PEG (Table 4). While soluble protein concentrations in controls and with K. arvensis were not affected, PEG increased the concentration of soluble protein in P. peltatum flasks, particularly at 1 h (Fig. 1). Consequently, the inhibition of end-product formation of proteolysis mediated by P. peltatum, H. canum, A. uva-ursi, A. unedo and E. arborea was prevented, at least partially, by the addition of PEG (Table 4) leading to increased concentrations of branched SCFA and NH3 after 12 h.
Table 4 Phenolic composition of plant samples, protein precipitating capacity (mg bovine serum albumin (BSA)) precipitated per g DM sample*

* For details of procedures, see Materials and methods.
† Below detection limit.
Discussion
The ’Rumen-up’ collection of plants and plant extracts consists of 450 samples of plant material, mainly foliage, and fifty essential oil compounds. Broadly speaking, the samples were considered to be possible candidates for manipulating ruminal fermentation based on traditional uses, known phytochemical composition, agronomic properties and combinations of these factors. The collection was restricted to plants that grew or could be grown in one of the countries of the European Union. Thus, although a wide range of plant types was collected, the collection cannot be considered in any way to be representative of botanical diversity. Specimens were taken from different geographical locations, under different weather conditions and at different times of the year. Phytochemical concentrations in plant tissues are subject to change by all these factors (Wink, Reference Wink and Wink1999). For example, the formation of steroidal saponins varied 13–14-fold in different samples of Narthecium ossifragum depending on location and time (Flåøyen et al. Reference Flåøyen, di Menna, Wilkins, Sandvik and Berndt2004). Furthermore, sample processing consisted of freeze-drying and storage for, in some cases, many months, which would influence the survival of volatile or labile compounds. Therefore, many possible positive samples could have been overlooked because they were collected at the wrong place or time, or were processed in an inappropriate way. Nevertheless, the results of the screening programme are indicative of the potential value of plants and their extracts as modifiers of ruminal fermentation.
The initial screening for samples inhibitory to ruminal proteolysis used a 30-min incubation of ruminal digesta with radiolabelled casein. No substrate was added that would support microbial growth. The assay was therefore a simple short-term determination of enzyme activity. Subsequently, an adaptation of the batch culture system developed by Mauricio et al. (Reference Mauricio, Mould, Dhanoa, Owen, Channa and Theodorou1999) was used to investigate the most promising samples. The addition of BSA and soya proteins at the concentrations used here was the culmination of development work that established the most appropriate incubation times, proteins to add and the concentrations of the proteins to be added. A mixture of 2 mg/ml soyabean flour and 0·13 mg/ml BSA was chosen as protein supplement leading to a final crude protein content of 169 g/kg in the substrate (Table 1), which is well within the range of practical diets. These concentrations further allowed complete degradation of soluble protein within 9–16 h. Both protein sources yielded identifiable banding patterns for qualitative and quantitative analysis by PAGE. The most significant difference with the batch culture system was that it had the considerable advantage that microbial growth was a component of the incubation. Thus, samples that decreased proteolytic activity by suppressing the growth of certain bacteria involved in protein degradation, in a manner similar to the feedlot ionophore, monensin, (Yang & Russell, Reference Yang and Russell1993) could be identified. The batch culture system also enabled measurements of protein breakdown products, including branched SCFA and NH3. This capability reinforced direct measurements of proteolysis but also produced additional information about possible effects on deamination of amino acids.
Five of the samples identified in the initial screening programme retained their activity in the batch culture system. These samples, P. peltatum, H. canum, E. arborea, A. unedo and A. uva-ursi, were characterized by a high content of phenolic compounds in general and high biological protein precipitating capacities. Their tannin content and specific protein precipitating capacity varied, but the total tannin concentration corresponded reasonably well to the extent of inhibition of proteolysis in batch culture. Furthermore, the reversal of inhibition by PEG indicated that tannins were responsible for the inhibition. Tannins have long been known to inhibit protein digestion. Conversion of protein to an insoluble form less susceptible to proteinase activity is generally considered to be the mechanism by which they work (Molan et al. Reference Molan, Attwood, Min and McNabb2001). However, the present study illustrates that the ability to precipitate the model protein BSA is alone not sufficient to ensure effectiveness with other protein sources in a mixed incubation. E. arborea was most effective of all samples in precipitating BSA, but had a minor influence on proteolysis as determined by two independent methods using casein or soya meal as protein sources. It is possible that, in the protein–tannin complex formed, the protein remained susceptible to microbial digestion. Alternatively, the precipitating material may have been labile to microbial digestion. Nevertheless, tannins are highly heterogeneous in structure and the interaction of different tannins with protein may yield complexes that vary in their protection in the rumen and subsequent ability to release the protein in the small intestine. Their effectiveness nutritionally may therefore differ in a similar manner. The use of tannin-rich feeds, although promising in some trials, has often not yielded the anticipated nutritional benefits in in vivo trials (Waghorn et al. Reference Waghorn, Ulyatt, Johns and Fisher1987; Barry & McNabb, Reference Barry and McNabb1999, Poncet & Rémond, Reference Poncet and Rémond2002). Increased abomasal protein flows induced by Lotus pedunculatus were counteracted by a decreased apparent digestibility in the small intestine, resulting in only a small increase in apparent absorption of essential amino acids (Waghorn et al. Reference Waghorn, Shelton, McNabb and McCutcheon1994). These results were attributed to either the tannins not releasing amino acids in the small intestine or to their inactivating digestive enzymes. One of the greatest problems with tannins is that they interact with microbial enzymes as well as feed proteins, resulting in decreased fermentative activity (Reed et al. Reference Reed, McDowell, Van Soest and Horvath1982; Makkar et al. Reference Makkar, Singh and Dawra1988b). The tannin-rich samples investigated here all gave decreased gas production over 12 h. SCFA production was unaffected, however, so the samples appear to merit further investigation as potential feed additives.
K. arvensis had entirely different properties. The inhibition of proteolysis was not mediated by tannins, since the tannin content of the plant sample was very low and the effect was not reversible by the addition of PEG. Several components of plant tissues other than tannins are known to inhibit proteolysis. Some inhibitors, such as soyabean trypsin inhibitor, are polypeptides (Beynon & Salvesen, Reference Beynon, Salvesen, Benyon and Bond1989). Others are enzymes that release inhibitory compounds. Polyphenol oxidase converts phenols into quinones, which then react rapidly with proteins or proteolytic enzymes, an activity that leads to decreased degradation rates of substrate protein. This activity causes improvements in protein survival during the ensiling of red clover in comparison to alfalfa and other clover species (Jones et al. Reference Jones, Muck and Hatfield1995) and a greater rumen escape was estimated for red clover proteins (Broderick & Albrecht, Reference Broderick and Albrecht1997). The agent in K. arvensis responsible for decreasing proteolysis seems unlikely to be polypeptide in nature, however, because activity was retained following treatment of the plant material with hot petroleum ether at 90°C (N. Selje, E. M. Hoffman & K. Becker, unpublished results). Saponins also improve N retention by intervening in microbial proteolytic activity. It is not a direct effect on proteolysis, however, but a suppression of the bacteriolytic activity of protozoa (Eugène et al. Reference Eugène, Archimede and Sauvant2004). Few reports exist on the protein-binding capacities of saponins and effects on enzyme activities that seem highly dependent on the quality of saponins and proteins (Potter et al. Reference Potter, Jiminez-Flores, Pollak, Lone and Berber-Jiminez1993, Ikedo et al. Reference Ikedo, Shimoyamada and Watanabe1996, Shimoyamada et al. Reference Shimoyamada, Ikedo, Ootsubo and Watanabe1998). In continuous culture, extracts of yucca (8 % sarsaponin) led to an accumulation of peptide-N, which was attributed to either a stimulation of proteolysis or an inhibition of peptidolysis (Cardozo et al. Reference Cardozo, Calsamiglia, Ferret and Kamel2004).
The active ingredient in K. arvensis appears more likely to be a selective inhibitor of bacterial growth rather than a protease inhibitor. In some ways, the effect resembled that of monensin. The effects of monensin in the batch culture system were similar to those described from other in vitro and in vivo studies (Van Nevel & Demeyer, Reference Van Nevel and Demeyer1977; Schelling, Reference Schelling1984; Jalč & Lauková, Reference Jalč and Lauková2002). The greatest effect of monensin was a decreased degradation of soluble protein, which was accompanied by a decrease in the concentration of end products, in particular of branched SCFA. Monensin is an ionophore that selectively inhibits the growth of Gram-positive bacteria (Chen & Wolin, Reference Chen and Wolin1979) and leads to adaptive changes in some Gram-negative species (Callaway & Russell, Reference Callaway and Russell1999), both of which contribute to a slowing of NH3 formation from protein. Some phytochemicals may have similar effects. McIntosh et al. (Reference McIntosh, Williams, Losa, Wallace, Beever and Newbold2003) reported on the inhibitory effects of an essential oil preparation on deamination of amino acids in ruminal digesta in vitro, an effect that was attributed to a selective inhibition of obligately peptidolytic Gram-positive species, Clostridium, Peptostreptococcus and Eubacterium. Preliminary analysis by HPLC indicated no substantial content of common essential oils in K. arvensis (R. Losa, personal communication). Further selective extraction and chromatography requires to be done to identify the active ingredient.
The magnitude of the proteolysis-inhibiting effect of monensin was greater than that of K. arvensis; the former, however, being a pure substance in contrast with a complex plant material. Extraction of the plant material will narrow down the spectrum of added compounds and avoid overlaps with nutritive effects of the plant samples. Testing of these extracts in the batch culture system should lead to a more distinctive impact on protein degradation.
A patent was filed (patent no. WO2005099729) for the application of K. arvensis and P. peltatum in ruminant nutrition.
Conclusion
Screening a targeted collection of 500 plants and plant extracts for effects on rumen microbial proteolysis revealed five samples that were particularly promising. Four were rich in tannins. These samples had no inhibitory influence on SCFA production, indicating that they may be less problematic in slowing fermentation than some other tannin-containing plants. Gas production was inhibited slightly, however, so further studies are required to determine the usefulness of these samples in vivo. The fifth sample, K. arvensis, had a different mode of action that seems to be particularly promising, in that it is different from any reported previously for inhibiting ruminal proteolysis and did not appear to cause any inhibition of fermentation. It is important now to elucidate the underlying mechanism of inhibition, its persistence in long-term culture experiments, the precise chemical nature of the active ingredient and to validate its nutritional usefulness in vivo. K. arvensis may eventually form the basis of alternative feed additives/ingredients that inhibit ruminal proteolysis and improve protein nutrition in ruminants.
Acknowledgements
The authors thank C. Apel, H. Baumgärtner and B. Fischer for excellent technical support and Dr E. Schlecht and Dr H. P. S. Makkar for critical reading of the manuscript. We are particularly grateful to Dr H. Steingaß, Institute of Animal Nutrition, University of Hohenheim, for access to cannulated cows. This work was part of the project RUMEN UP supported by the European Union commission (QLK5-CT-2001-00 992). We thank all partners of the consortium (H. Makkar, D. Beever, F. Mould, B. Morgan, K. Kliem, S. López, J. Gonzalez, C. Duffy, R. Losa, M. Frehner) for cooperation and valuable discussion. The Rowett Research Institute is funded by the Scottish Executive Environment and Rural Affairs Department.









