Pre-diabetes is a condition of abnormal glucose homoeostasis such as impaired fasting glucose, impaired glucose tolerance (IGT) or a combination of both( Reference Nathan, Davidson and DeFronzo 1 ). This disorder is diagnosed by the fasting blood glucose (FBG) test with values between 5·6 and 6·9 mmol/l being regarded as pre-diabetic or by the 2-h oral glucose tolerance test (OGTT) with values between 7·8 and 11 mmol/l or glycosylated Hb (HbA1c) of 5·7–6·4 % being regarded as pre-diabetic( 2 ). Pre-diabetes is associated with lack of physical activity, abdominal obesity, dyslipidaemia and hypertension, and can lead to the development of insulin resistance, diabetes, chronic kidney disease and CVD( Reference Eldin, Emara and Shoker 3 – Reference Al-Sinani, Al-Shafaee and Al-Mamari 5 ). The prevalence of pre-diabetes has been reported to be 28·7 % based on FBG and 12·4 % based on HbA1c in the USA( Reference Middelbeek and Abrahamson 6 ). In 2008, about 7·7 % of the Iranian population was diagnosed with diabetes, and 16·8 % was diagnosed with pre-diabetes by the FBG test( Reference Esteghamati, Gouya and Abbasi 7 ). In the past few decades, the prevalence and incidence of type 2 diabetes have increased, and the disease has therefore become an epidemic around the world. In Asia, the rate of increase shows no sign of slowing down( Reference Whiting, Guariguata and Weil 8 , Reference Yoon, Lee and Kim 9 ). It has been reported that diabetes can be prevented through lifestyle modification in individuals who are at high risk of type 2 diabetes( Reference Lindström, Ilanne-Parikka and Peltonen 10 , Reference Li, Zhang and Wang 11 ). Western lifestyle, characterised by reduced physical activity and consumption of foods rich in SFA, refined grains and red meat, has been reported to increase the risk of diabetes( Reference Feskens, Virtanen and Rasanen 12 ). Identifying dietary patterns and finding their relationship with chronic diseases is better than studying the relationships between nutrients and diseases; this is because people do not make use of the concept of nutrients and food separately. In addition, the nutrients of different foods may have synergistic or interactive effects on each other and it is easier to educate subjects on dietary patterns( Reference Hu 13 ). Most available studies have examined the relationship between dietary patterns and diabetes( Reference Maghsoudi and Azadbakht 14 ), but studies investigating the associations between dietary patterns and pre-diabetes are limited( Reference Mizoue, Yamaji and Tabata 15 , Reference He, Ma and Zhai 16 ). Moreover, the increasing incidence of diabetes and unique patterns of food consumption among the Iranian population( Reference Amini, Shafaeizadeh and Zare 17 ), therefore, make it interesting to assess the relationship between dietary patterns and pre-diabetes. Most previous studies in Iran have only reported the dietary patterns of healthy subjects( Reference Rezazadeh, Rashidkhani and Omidvar 18 , Reference Esmaillzadeh and Azadbakht 19 ). However, a cross-sectional study in one of the cities of Iran found an inverse association between vegetarian dietary patterns and IGT( Reference Amini, Shafaeizadeh and Zare 17 ). The aim of the present study was therefore to determine the relationship between dietary patterns and pre-diabetes using a matched case–control study design.
Methods
Subjects
A matched case–control study design was used to investigate 300 subjects, who attended the diabetes screening centre in Shahreza, Iran, from May to October, 2014. Subjects >30 years of age and who were at risk of diabetes morbidity based on the existence of at least one of the following criteria were referred to this centre: being overweight or obese, having a family history of diabetes or existence of at least two symptoms of diabetes. The diagnosis of pre-diabetes among subjects was made at the clinic. This study included 150 subjects who had been diagnosed with pre-diabetes (cases). After recruitment of all cases, 150 healthy subjects with normal FBG, considering other inclusion criteria, were recruited as the control group. Using the frequency matching method, the two groups were matched by age and sex. The groups matched for age were as follows: 35–44, 45–54 and 55–65 years; the two groups were also matched for sex. Inclusion criteria for the case group included subjects aged 35–65 years, FBG between 5·6 and 6·9 mmol/l or 2-h OGTT of 7·8–11 mmol/l, and diagnosis confirmed at least 3 months before the start of the study. Inclusion criteria for the control group included subjects aged 35–65 years, FBG<5·6 mmol/l and 2-h OGTT<7·8 mmol/l during screening. However, subjects who were found and/or reported to be alcoholics, drug addicts (current use or stopped within 3 months before the study), smokers or used any tobacco products, such as a smoking of pipe at least once a week, were excluded from the study. Subjects with BMI≥40 kg/m2, pregnant or lactating women, those on a special diet during the previous year, subjects with diagnosed heart disease, diabetes, hypertension, dyslipidaemia, renal or hepatic impairment and multiple sclerosis, and individuals on drugs were also excluded. Dietary supplement intake was defined as daily intake of supplements in the previous month. Written informed consent was obtained from all participants before commencement of the study. The study protocol was approved by the ethics committee of Tehran University of Medical Sciences.
Assessment of anthropometry, blood pressure and physical activity
Anthropometric measurements such as weight, height, waist circumference (WC), as well as systolic and diastolic blood pressure were obtained from all participants. Weight was measured to the nearest gram (100 g) without shoes and with minimal clothing using the Seca scale. Height was measured to the nearest 0·1 cm while standing without shoes using the Seca stadiometer (Seca 216). WC was measured using a flexible tape at the midpoint between the lowest rib and the iliac crest hip. Systolic blood pressure and diastolic blood pressure were measured once in the left arm while sitting using a manual sphygmomanometer. The BMI of each participant was calculated by dividing the weight in kilograms by the square of height in metres. Abdominal obesity was defined as WC≥80 cm in women and WC≥90 cm in men( 20 ). General obesity was defined as BMI≥30 kg/m2. Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ)( 21 ). The information collected using IPAQ was categorised as ‘low physical activity’ (point score <600 metabolic equivalent (MET)/h per week), ‘moderate physical activity’ (point score between 600 and 3000 MET/h per week) and ‘high physical activity’ (point score>3000 MET/h per week).
Biochemical assessment
Blood samples were collected after an overnight fast (at least 8 h) for FBG measurement. The subjects underwent a 2-h OGTT. Venous blood was used, and plasma glucose was analysed at 546 nm wavelength using the photometric method (glucose oxidase method).
Dietary assessment
Dietary information was obtained by interview using a 168-item, semi-quantitative FFQ, which measured the previous year’s food intake (for cases the year before diagnosis of pre-diabetes and for controls during the previous year). The participants were asked how often they consumed each food item of different categories: daily, weekly, monthly or yearly. Information obtained from the FFQ was converted to g/d in order to estimate daily food intake. The validity and reliability of the FFQ used were tested( Reference Azadbakht and Esmaillzadeh 22 ).
Statistical methods
Statistical analysis was performed using Statistical Package for Social Sciences, version 16 (SPSS Inc.). On the basis of the similarity of food items and previous studies( Reference Esmaillzadeh and Azadbakht 19 , Reference Zarodi, Mirmiran and Fazel Tabar 23 ), twenty-seven food groups were created. A food item was categorised into an independent group if its nutrient content had a huge difference from other food items (such as eggs) or the consumption of such an item was a special food habit (such as pickles) (Table 1). Factor analysis was used to determine the dietary patterns of all participants( Reference Jackson 24 ). In this study, principal component analysis with varimax rotation was used to determine the major dietary patterns, and then the factors were pre-selected to determine which set of factors meaningfully described the distinct dietary patterns. Factors with eigenvalues ≥1·8 were chosen as the major dietary patterns. Thereafter, a factor score was calculated for each participant by summing the intakes of food groups, weighted by their factor loading. Factor loading represents the correlation between a dietary pattern and each food group. A larger absolute value indicates greater correlation, and a positive or negative sign shows direct or inverse relationship, respectively, between the food group and dietary pattern.
Table 1 Food grouping used in the factor analysis

Factor loadings <0·3 were retained in the analysis, but removed from the table for simplicity (Table 1). The normality of distribution of the data was tested using the Kolmogorov–Smirnov test. The variables with normal and non-normal distribution were compared using the independent t and Mann–Whitney tests, respectively. The χ 2 test was used to compare the qualitative variables between cases and controls. To assess the correlation between dietary patterns and quantitative variables with normal and non-normal distribution, Pearson’s and Spearman’s correlation coefficients were used, respectively. Multivariate logistic regression analysis was performed to assess the relationship between pre-diabetes and dietary patterns. Statistical significance was set at P<0·05.
Results
In the present study, two dietary patterns were identified using factor analysis. The identified dietary patterns were the vegetables, fruits and legumes (VFL) dietary pattern, characterised by high intakes of vegetables, legumes, dried fruits, tomatoes, fruits, low-fat dairy products, chicken, vegetable oil, whole grains, nuts and fish and low intakes of refined grains, and the sweet, solid fat, meat and mayonnaise (SSMM) dietary pattern, characterised by high consumption of sweets and desserts, solid fats, red meat and processed meat, mayonnaise, high-fat dairy products, fried potato, compote, pickles, pizza, eggs, soft drinks, cooked potato, tea and coffee, salt, chicken, liver and organ meat. The factor scores were categorised as tertiles. This showed that in the VFL pattern the first tertile was less healthy, the second was moderate and the third was the healthiest. A similar interpretation was applied to the SSMM pattern. The total variance for the identified dietary patterns was 27·7 % (Table 2). The general characteristics and comparison of the subjects in the case and control groups are shown in Tables 3 and 4. The mean years of education, blood pressure, FBG, 2-h OGTT, weight, WC, BMI, energy intake, the percentage of abdominal and general obesity and low physical activity were higher in subjects with pre-diabetes as compared with the control group (P<0·001). No significant difference was, however, observed between the two groups with regard to the other characteristics.
Table 2 Factor loading for major dietary patternsFootnote *
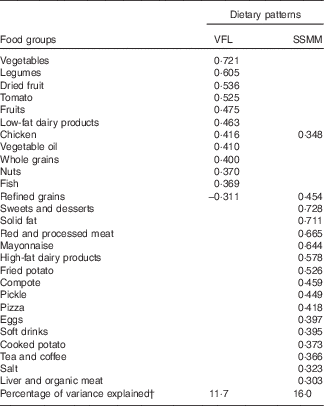
VFL, vegetables, fruits and legumes; SSMM, sweet, solid fat, meat and mayonnaise.
* Values <0·3 were excluded from the table for simplicity.
† Kaiser–Meyer–Olkin measure of sampling adequacy=0·751. Bartlett’s test of sphericity<0·001.
Table 3 Characteristics of the study participants across case and control groups (Mean values and standard deviations)

* Mann–Whitney test.
† Independent t test.
Table 4 Characteristics of the study participants across case and control groups (Numbers and percentages)

MET, metabolic equivalent.
* χ 2 test.
† <600 MET/h per week: ‘low’, 600–3000 MET/h per week: ‘moderate’, >3000 MET/h per week: ‘high’.
The relationship between the general characteristics and the dietary patterns is shown in Table 5. The data show that subjects in the third tertile of the VFL dietary pattern had lower weight, WC, BMI, energy intake, diastolic blood pressure, fasting blood sugar and 2-h OGTT compared with those in the first tertile. Furthermore, those in the third tertile of the SSMM dietary pattern were found to have higher educational level, weight, WC, BMI, systolic and diastolic blood pressures, FBG and 2-h OGTT compared with those in the lowest tertile. The VFL dietary pattern score negatively correlated with weight, WC, BMI, energy intake, diastolic blood pressure, FBG and 2-h OGTT (P<0·03). The SSMM dietary pattern score positively correlated with education, weight, WC, BMI, systolic and diastolic blood pressures, FBG and 2-h OGTT (P<0·002).
Table 5 General characteristics of the participants across tertiles of dietary pattern (Numbers and percentages; mean values and standard deviations; n 100)

VFL, vegetables, fruits and legumes; SSMM, sweet, solid fat, meat and mayonnaise.
* χ 2 test.
† P value is for Spearman’s correlation test.
‡ P value is for Pearson’s correlation test.
The OR for pre-diabetes across dietary patterns are shown in Table 6. The scores of the VFL dietary pattern were associated with lower pre-diabetes morbidity (OR 0·16; 95 % CI 0·10, 0·25). After adjusting for age, education, physical activity, BMI and energy intake in model 3, the relationship remained significant (OR 0·16; 95 % CI 0·10, 0·26). The OR of pre-diabetes was positively associated with the SSMM dietary pattern (OR 3·01; 95 % CI 2·13, 4·26), and the OR was found to be higher even after controlling for possible confounding variables (OR 5·45; 95 % CI 3·22, 9·23).
Table 6 Association between dietary patterns and pre-diabetes morbidity (Odds ratios and 95 % confidence intervals)
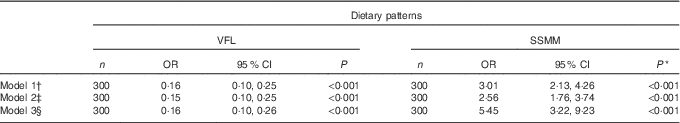
VFL, vegetables, fruits and legumes; SSMM, sweet, solid fat, meat and mayonnaise.
* Multivariate logistic regression analysis.
† Crude model.
‡ Adjusted for age, education, physical activity and BMI.
§ Additionally adjusted for energy intake.
Discussion
In this study, the association between dietary patterns and pre-diabetes morbidity was investigated. The results show that the VFL and SSMM dietary patterns were inversely and directly related to pre-diabetes morbidity, respectively. In addition, the VFL dietary pattern was inversely related to weight, WC, BMI, energy intake, diastolic blood pressure, FBG and 2-h OGTT. Furthermore, the SSMM dietary pattern was positively associated with weight, WC, BMI, systolic and diastolic blood pressures, FBG and 2-h OGTT.
In a study on women in Tehran, the results showed that Healthy and Western dietary patterns were inversely and positively related to insulin resistance, respectively( Reference Esmaillzadeh, Kimiagar and Mehrabi 25 ). A study on Japanese men also found that a dietary pattern high in dairy products, fruits, vegetables and starch and low in alcohol was related to a lower risk of IGT. However, those who followed the Japanese dietary pattern, which is characterised by high intakes of soyabeans, green tea, sea alga, pickles, vegetables and fish, were found to have high risk of IGT. Meanwhile, in other studies, some of the food items found in the Japanese dietary pattern were also reported in Healthy dietary patterns, and therefore further studies are suggested to clarify the relationship between Japanese dietary patterns and pre-diabetes in the Japanese population( Reference Mizoue, Yamaji and Tabata 15 ). In a study conducted in China, IGT was highly associated with the New Affluence dietary pattern. Subjects following this dietary pattern had certain lifestyle and food intake patterns, such as living in urban areas, had lower physical activity, were overweight and had higher intakes of animal food and soyabeans( Reference He, Ma and Zhai 16 ).
A meta-analysis of ten large prospective studies showed that adherence to a Healthy dietary pattern was associated with reduced risk of developing type 2 diabetes( Reference Esposito, Kastorini and Panagiotakos 26 ). Dietary patterns characterised by high consumption of fruits and vegetables, whole grains, fish and poultry and low consumption of red meat, processed foods, sugar-sweetened beverages and starchy foods may retard the progression of type 2 diabetes( Reference Esposito, Kastorini and Panagiotakos 26 ). In a study on type 2 diabetic patients, the results showed that dietary recommendations such as higher intakes of vegetables, nuts for snacks, fruits instead of juices, whole grains instead of processed cereals, legumes instead of potatoes and increased physical activity can provide long-term, sustained weight loss( Reference García de, Torre, del Valle and Durán 27 ). The results of another study on middle-aged subjects with IGT showed that intensive dietary intervention for reducing dietary total fat and SFA, and therefore an increase in dietary fibre, can prevent type 2 diabetes( Reference Lindström, Louheranta and Mannelin 28 ). In addition, the Mediterranean diet has been shown to be effective in the prevention of diabetes incidence in subjects with high cardiovascular disease risk( Reference Salas-Salvadó, Bullo and Babio 29 ).
The dietary patterns found in the present study are similar to those found in diabetic and healthy subjects in Iran( Reference Zarodi, Mirmiran and Fazel Tabar 23 , Reference Esmaillzadeh, Kimiagar and Mehrabi 25 , Reference Rezazadeh and Rashidkhani 30 ). The VFL dietary pattern found in the present study is also similar to the Healthy dietary patterns of other studies( Reference Mizoue, Yamaji and Tabata 15 , Reference Esposito, Kastorini and Panagiotakos 26 ) However, dietary patterns are only comparable when similar categorisations of food groups have been used and similar patterns of factor loadings have been found. The total variance for the identified dietary pattern in this study was 27·7 %, which is almost similar to the total variance of other studies( Reference Esmaillzadeh and Azadbakht 19 , Reference Zarodi, Mirmiran and Fazel Tabar 23 , Reference Esmaillzadeh, Kimiagar and Mehrabi 25 , Reference Amini 31 ).
It has been reported that healthy diets with high intakes of vegetables, vegetable oil, fish, fruits and nuts have food compounds or nutrients such as fibre, vitamins and minerals, which may decrease diabetes through a decrease in inflammation, improved metabolism of glucose and endothelial function, and sensitivity to insulin( Reference Feskens, Virtanen and Rasanen 12 , Reference Hu, Van Dam and Liu 32 ).
The positive relationship found between the SSMM dietary pattern and pre-diabetes could be a result of low intakes of healthy foods such as fruits and vegetables and high intakes of foods such as red meat, processed meat and animal fat. The identified dietary patterns high in red meat and processed meat have been related to diabetes morbidity( Reference Micha, Wallace and Mozaffarian 33 – Reference Aune, Ursin and Veierød 35 ). Processed meat contains a lot of salt and nitrite; moreover, a decrease in insulin action with higher Na intake has been reported( Reference Donovan, Solomon and Seely 36 ). Nitrite changes to nitrous amine, which is poisonous to the β cells of the pancreas, and leads to insulin resistance and glucose intolerance( Reference Aune, Ursin and Veierød 35 ). In addition, meat is an important source of SFA. High intake of SFA has been shown to be associated with high risk of pre-diabetes and glucose intolerance( Reference Thanopoulou, Karamanos and Angelico 37 ). In this study, the low dietary fibre intake observed in the SSMM dietary pattern could have also played a role in the risk of pre-diabetes, as studies have shown that dietary patterns high in fibre (specially fibre of grains) protect against diabetes( Reference Al-Khudairy, Stranges and Kumar 38 ). The high intake of whole grains, legumes, vegetables and fruits containing high fibre, owing to their low energy content and high satiety effect, may decrease food intake and weight gain( Reference Al-Khudairy, Stranges and Kumar 38 ). However, the inverse relationship between fibre and risk of diabetes is not clear. It seems that viscose fibre lowers the speed of gastric evacuation and absorption in the small intestine( Reference Hu, Van Dam and Liu 32 , Reference Al-Khudairy, Stranges and Kumar 38 ).
In the present study, the SSMM dietary pattern was observed to be characterised by high consumption of cooked potatoes, refined grains, sweets and desserts. These foods seem to have high glycaemic indices and their load could be of concern. There are reports of a direct relationship between glycaemic load and type 2 diabetes( Reference Schulze, Liu and Rimm 39 ), and although the exact mechanism is not clear there are some suggested approaches. High amounts of carbohydrate with high glycaemic load have been reported to increase the glucose level of the blood and the demand for insulin. Besides, high demand for insulin has been demonstrated to have some effects on the pancreas and could gradually lead to glucose intolerance( Reference Schulze, Liu and Rimm 39 ). Several studies have shown the relationship between sweets and sugar intake and risk of type 2 diabetes( Reference Kastorini and Panagiotakos 40 ). It has been reported that high intakes of refined sugar and sweet drinks with high glycaemic load could lead to an increase in weight gain, which can affect the metabolism of glucose and sensitivity to insulin, and could eventually increase the risk of diabetes( Reference Al-Khudairy, Stranges and Kumar 38 ). In this study, tea, coffee and mayonnaise were included in the SSMM dietary pattern, although some studies have shown that the consumption of tea and coffee has protective effect on diabetes( Reference Salazar-Martinez, Willet and Ascherio 41 ); however, in some other studies, both featured in Unhealthy dietary patterns( Reference Zarodi, Mirmiran and Fazel Tabar 23 , Reference Esmaillzadeh, Kimiagar and Mehrabi 25 ), and this might be because of the intake of sugar and sweets along with tea or coffee. Mayonnaise has high-fat, creamy sauce( Reference Hu, Rimm and Smith-Warner 42 ), and in some studies it features in the Western dietary pattern( Reference Esmaillzadeh, Kimiagar and Mehrabi 25 , Reference Amini 31 , Reference Hu, Rimm and Smith-Warner 42 ). In this study, chicken was included in both dietary patterns, which might be attributed to its high consumption in Iran.
Furthermore, in this study, the mean education level was higher in pre-diabetic subjects compared with the control participants. In a study in Bangladesh, the results showed that high education level was positively related to the risk of both diabetes and pre-diabetes( Reference Akter, Rahman and Abe 43 ). It has been reported that education and wealth increases the risk of obesity( Reference Monteiro, Conde and Popkin 44 ), which is a risk factor for pre-diabetes( Reference Al-Shafaee, Bhargava and Al-Farsi 45 ). However, several studies in Iran( Reference Harati, Hadaegh and Saadat 46 – Reference Golozar, Khademi and Kamangar 48 ), Spain( Reference Soriguer, Goday and Bosch-Comas 49 ) and the USA( Reference Borrell, Dallo and White 50 ) have found an inverse association between diabetes morbidity and level of education. Therefore, further investigation is required in this regard.
The present study has some limitations. First and foremost, the dietary patterns were obtained from food intake patterns using a validated FFQ, although food consumption behaviours such as pattern, time, number of meals and ways of preparing the food are also important( Reference Tseng 51 ). Second, a questionnaire was used to collect dietary information and as such there is the possibility of error because the information collected depends largely on recall. Third, despite the fact that pre-diabetes subjects were asked to report their dietary intake of the year before the diagnosis of pre-diabetes, they would have received dietary advice after diagnosis, which might affect their dietary responses. Fourth, factor analysis was used to identify the dietary patterns, and as such food categorisation usually can change on the basis of the researchers’ interest. Fifth, although pre-diabetes was not observed in the controls, they were referred to our centre because of the existence of some criteria such as being overweight or obese, having a family history of diabetes or existence of at least two symptoms of diabetes. Thus, the comparison between pre-diabetics and controls could potentially not reflect a comparison between cases and true controls. Sixth, similar to all case–control studies, this study could not determine the temporal relationship between dietary patterns and pre-diabetes. However, despite the aforementioned limitations, the present study is one of the few studies that have examined the relationship between dietary patterns and risk of pre-diabetes using a case–control study design.
In conclusion, the VFL and SSMM dietary patterns are inversely and directly related to pre-diabetic morbidity, respectively. In addition, the VFL dietary pattern is associated with risk of obesity and hypertension, which are implicated in impaired glucose metabolism and development of diabetes and CVD. Future studies are therefore needed to confirm our findings with other populations.
Acknowledgements
The authors sincerely express their appreciation to the participants of this study.
This research project was supported by Tehran University of Medical Sciences (TUMS) (grant no. 93-454-76). TUMS had no role in the design, analysis or writing of this article.
G. S. and F. S. conceived and developed the idea for the study and revised the manuscript; F. B., O. M. S. and Parvaneh Yavari contributed to data collection. F. B. wrote numerous drafts of the manuscript; B. M., Mostafa Qorbani and F. K. contributed to data analysis and interpretation of the data.
There are no conflicts of interest.











