The capacity of fish to metabolise carbohydrate (CH) varies among species, and it is dependent on particular characteristics such as their feeding habits( Reference Hemre, Mommsen and Krogdahl 1 – Reference Polakof, Panserat and Soengas 3 ). Herbivorous or omnivorous fish species such as tilapia (Oreochromis niloticus) or bagrid catfish (Mystus nemurus) have been reported to respond favourably to elevated levels of dietary CH showing propitious growth associated with an adequate enzymatic response( Reference Azaza, Khiari and Dhraief 4 , Reference Hamid, Mahayat and Hashim 5 ). Carnivorous fish species do not generally tolerate high CH in the diet( Reference Hemre, Mommsen and Krogdahl 1 – Reference Polakof, Panserat and Soengas 3 ). However, there is some variation in this capacity( Reference Stone 2 ). Thus, some carnivorous species are able to efficiently use diets with CH inclusion levels about 20–30 %, as reported for sea bass (Dicentrarchus labrax)( Reference Enes, Panserat and Kaushik 6 , Reference Peres and Oliva-Teles 7 ), cobia (Rachycentron canadum)( Reference Mingchun, Qinghui and Kangsen 8 ) or yellow croaker (Larmichthys crocea)( Reference Piaoping, Mengqiang and Fengjun 9 ). In contrast, other carnivorous fish species such as rainbow trout (Oncorhynchus mykiss) or Atlantic salmon (Salmo salar) indicated deleterious effects of high dietary CH in terms of declined growth or metabolic disorders such as hepatomegaly( Reference Aksnes 10 , Reference Krogdahl, Sundby and Olli 11 ), which have been associated with anomalies in glucose metabolism in these species( Reference Panserat, Medale and Breque 12 – Reference Panserat, Capilla and Gutierrez 14 ). These metabolic capabilities are associated with a better tolerance to glucose in herbivorous or omnivorous species, indicated by a higher clearance rate of glucose after performing a glucose tolerance test (GTT)( Reference Polakof, Panserat and Soengas 3 ). Furthermore, the tolerance to glucose in fish is influenced by other macronutrients present in the diet such as protein or lipids( Reference Figueiredo-Silva, Panserat and Kaushik 15 , Reference Borges, Valente and Véron 16 ).
The metabolic utilisation of dietary CH in fish relies mainly on the activity of hepatic enzymes, which can be modulated by nutritional conditions( Reference Enes, Panserat and Kaushik 17 ). The nutritional modulation of enzymes involved in glucose metabolism varies among different species, and even within single species, depending on several factors such as temperature, age or nutritional background( Reference Hemre, Mommsen and Krogdahl 1 , Reference Enes, Panserat and Kaushik 17 ). Thus, increased activity of glycolitic enzymes such as glucokinase (GK), 6-phosphofructo 1-kinase and pyruvate kinase (PK) were found in the liver of different fish species such as rainbow trout, gilthead seabream (Sparus aurata), common carp (Cyprinus carpio) or European sea bass (D. labrax) fed diets containing high levels of CH( Reference Panserat, Blin and Medale 18 , Reference Enes, Panserat and Kaushik 19 ). No such response was found in the muscle of carnivorous fish species( Reference Polakof, Panserat and Soengas 3 , Reference Kirchner, Seixas and Kaushik 20 ) in contrast to what is observed in omnivorous fish species( Reference Capilla, Medale and Panserat 21 ). Furthermore, enzymes involved in endogenous glucose production in liver are not affected by dietary CH content in most fish species studied( Reference Panserat, Medale and Breque 12 , Reference Panserat, Capilla and Gutierrez 14 , Reference Enes, Panserat and Kaushik 19 ), whereas an inhibition of gluconeogenesis was observed in common carp and gilthead seabream( Reference Panserat, Plagnes-Juan and Kaushik 22 ). Moreover, enzymes involved in hepatic lipogenesis are poorly induced by CH in carnivorous fish( Reference Panserat, Skiba-Cassy and Seiliez 23 ). All these metabolic divergences, added to different digestive characteristics, distinguish fish capability to use dietary CH.
The dietary CH utilisation in fish is of great economical and environmental importance in the present context of aquaculture nutrition, as the alternative ingredients to the marine protein sources normally used in aquafeeds are obtained from vegetable sources, which contain high levels of CH( Reference Stone 2 ). In consequence, in the past years, several strategies able to modulate hepatic enzymes, such as the use of adaptation diets, genetic selection or the application of nutritional programming, have been used to improve CH utilisation in fish( Reference Jin, Medale and Kamalam 24 , Reference Geurden, Aramendi and Zambonino-Infante 25 ).
Senegalese sole (Solea senegalensis, Kaup 1858) is a flatfish with economical interest in European aquaculture( Reference Morais, Aragao and Cabrita 26 ). It has high protein requirement( Reference Rema, Conceição and Evers 27 ) and low capacity to utilise dietary lipids efficiently( Reference Borges, Oliveira and Casal 28 – Reference Borges, Medale and Veron 30 ). In terms of glucose tolerance, Conde-Sieira et al. ( Reference Conde-Sieira, Soengas and Valente 31 ) have recently reported that Senegalese sole is able to develop an efficient metabolic response under induced short-term hyperglycaemia, showing a good capacity to restore glucose homoeostasis. Furthermore, previous studies carried out with diets rich in CH suggest a good capacity of this species to use CH in terms of nutrient retention and growth potential( Reference Borges, Medale and Dias 29 , Reference Guerreiro, Peres and Castro 32 ) (Salas-Leiton et al., unpublished results). In a way similar to that observed in mammals and other fish species, glucose tolerance in Senegalese sole is impaired if fish are fed fat-enriched diets, resulting in prolonged hyperglycaemia and impaired insulin signalling( Reference Figueiredo-Silva, Panserat and Kaushik 15 , Reference Borges, Valente and Véron 16 ). Accordingly, Borges et al. ( Reference Borges, Valente and Véron 16 ) recommended a low lipid dietary inclusion to improve CH utilisation in this species, in contrast to the common aquaculture practices in which the use of fatty diets is usual for fish culture.
Taking all of this into account, this study aimed to evaluate in Senegalese sole the metabolic effect of feeding low-lipid diets with increasing protein:CH ratio at short- and long-term. The postprandial modulation of enzyme activities mainly related to glucose metabolism in liver and muscle, at short- and long-term, as well as the possible metabolic adaptation to dietary CH after a relatively long period of feeding CH-enriched diets were particularly focused. GTT were performed in fish 4 and 104 d after feeding four experimental diets containing increasing protein:CH content. In addition, the levels of metabolites in plasma (glucose, NEFA) and liver (glucose, glycogen, NEFA) were measured. Hepatic enzyme activities involved in glycolysis, glycogen metabolism, gluconeogenesis and lipogenesis were determined in the various dietary treatments. In muscle, both metabolite levels (glucose, glycogen, TAG) and hexokinase (HK) and glycogen phosphorylase (GPase) enzyme activities were assessed.
Methods
The experiments described comply with the Guidelines of the European Union Council on protection of animals (2010/63/EU) and were supervised by trained scientists following the Federation of European Laboratory Animal Science Associations category C recommendations.
Experimental diets
The experimental diets were isoenergetic (21 kJ/g DM), isolipidic (6 % DM) and contained four different dietary crude protein:total CH ratios: 48:38, 52:34, 56:30 and 60:26. The formulation and proximate composition of diets (Sparos) are presented in Table 1.
Table 1 Ingredients and proximate composition of the experimental diets

CP, crude protein; CF, crude fat.
* Danish fishmeal LT70: 71 % crude protein (CP), 11 % crude fat (CF) (Sopropêche).
† Super prime without guts: 84 % CP, 4·7 % CF (Sopropêche).
‡ Vital: 84 % CP, 1·3 % CF (Roquette).
§ Whole wheat: 10·2 % CP, 1·2 % CF (Casa Lanchinha).
|| Dehulled, microgrinded and extruded pea meal: 24 % CP, 0·4 % CF (Sotexpro).
¶ Vitamins (IU or mg/kg diet): dl-α-tocopheryl acetate, 100 mg; sodium menadione bisulphate, 25 mg; retinyl acetate, 20 000 IU; dl-cholecalciferol, 2000 IU; thiamin, 30 mg; riboflavin, 30 mg; pyridoxine, 20 mg; cyanocobalamin, 0·1 mg; nicotinic acid, 200 mg; folic acid, 15 mg; ascorbic acid, 1000 mg; inositol, 500 mg; biotin, 3 mg; calcium panthotenate, 100 mg; minerals (g or mg/kg diet): cobalt carbonate, 0·65 mg; copper sulphate, 9 mg; ferric sulphate, 6 mg; potassium iodide, 0·5 mg; manganese oxide, 9·6 mg; sodium selenite, 0·01 mg; zinc sulphate,7·5 mg; sodium chloride, 400 mg; calcium carbonate, 1·86 g; excipient wheat middlings (Premix Lda).
** Choline chloride 60 % (Premix Lda).
†† Betaine HCl 98 % (Premix Lda).
‡‡ Guar gum HV109 (Seah International).
§§ Paramega PX (Kemin Europe NV).
|||| Estimated by difference (100−ash−CP−CF).
Diet composition analysis was carried out in duplicate following the methodology described by the Association of Official Analytical Chemists (AOAC)( 33 ). Ash was analysed by combustion (550°C during 6 h) in a muffle furnace (Nabertherm L9/11/B170) and crude protein (N×6·25) using a Leco N analyser (model FP-528; Leco Corporation). Crude lipid content was determined by petroleum ether extraction (40–60°C) using a Soxtec™ 2055 Fat Extraction System (Foss), whereas starch followed Thivend et al.( Reference Thivend, Mercier and Guilbot 34 ). Gross energy was quantified in an adiabatic bomb calorimeter (Werke C 2000 basic; IKA).
Fish and rearing conditions
Senegalese sole juveniles were obtained from a fish farm (Aquácria Piscícolas, S.A.) where they were fed a commercial diet (9 % CH, 16 % lipids; 62 % crude protein), and transported to the experimental facilities of CIIMAR, Porto, Portugal where the growth trial was conducted. After arrival at the experimental unit, fish were kept under quarantine conditions for a 2-week period. Once acclimated to the new rearing facilities, 22 fish (22·01 (sem 0·01) g)/dietary condition were individually weighed, measured and distributed (initial stocking density of 1·65 kg/m2) among twelve fibreglass rectangular tanks (0·5 m×0·4 m) in a closed recirculation system. The system was supplied with filtered and heated (20·0 (sem 1·0)°C) saltwater (24 0/00) at a flow rate of 1·5 l/min. Total ammonium, nitrite, nitrate and pH levels were measured during the entire trial to ensure levels within the recommended ranges for marine species. Dissolved oxygen level was kept above 90·0 %±1·0 saturation. An artificial photoperiod of 12 h light–12 h dark was established. Triplicate groups of fish were fed to satiety with each dietary treatment using automatic feeders that distributed 8–10 meals/d during 104 d. The meal size offered to each tank was daily adjusted according to observations by an experienced researcher and based on the previous presence/absence of uneaten feed in each tank( Reference Borges, Oliveira and Casal 28 , Reference Cabral, Fernandes and Campos 35 ). By the end of the feeding trial and after the 24-h fasting period, soles were individually weighed (g) and measured (total length, cm). Daily Growth Index (DGI) was calculated as follows: 100×((W1)1/3−(W0)1/3)/d, where W0 and W1 are the initial and the final fish mean weights (g). Feed conversion ratio (FCR) was calculated as the amount of dry food intake (g)/weight gain (g). Voluntary feed intake (VFI) (g or kJ/kg average body weight (ABW) per d) was calculated as cumulative dry feed consumption/ABW per d, where ABW (kg) was (W1+W0)/2. The retention (% intake) was calculated as follows: 100×(W1×final carcass nutrient or energy content−W0×initial carcass nutrient or energy content)/total nutrient or energy intake (g or kJ/kg ABW per day).
Glucose tolerance test
GTT were performed at the initial and the final phases of the experiment (4 and 104 d after the beginning of the growth trial, respectively). With this purpose, thirty-two fish per dietary condition were distributed among eight tanks (two tanks/dietary condition) and maintained in the same conditions as described above. Senegalese sole fasted for 24 h were anaesthetised with MS-222 (100 mg/l) and immediately weighed (mean weight 25·76 (sem 0·47) g at the initial phase and 56·79 (sem 1·15) g at the final phase) for oral administration of 10 ml/kg fish of a saline solution with 1 g/kg of d-glucose. This concentration was selected according to previous studies( Reference Polakof, Míguez and Soengas 36 ) and successfully used also in Senegalese sole( Reference Conde-Sieira, Soengas and Valente 31 ). After oral administration, fish were placed in individual tanks according to different diets and sampling times (one tank per diet and per sampling point). Before glucose administration and after 1, 5 and 10 h, eight fish per dietary treatment were removed from the corresponding tank, anesthetised as above and blood sampled by caudal puncture with ammonium-heparinised syringes. Plasma samples were obtained after blood centrifugation, followed by deproteinisation with 0·6 m-perchloric acid and neutralisation with 1 m-potassium bicarbonate, frozen on dry ice and stored at −80°C until further assay of glucose.
Postprandial metabolic sampling
Both at the initial (4 d after the beginning of the growth trial) and at the final phases (104 d) of the experiment, tissue samples of fish from all experimental diets were taken at different postprandial times in order to analyse metabolic parameters. Thus, eight fish of each experimental diet were fed once to satiety and sampled just before feeding and 1, 5 and 10 h after the single meal distribution. On each sampling time, fish were removed from holding tanks, anaesthetised as above, and weighed. At each postprandial sampling time, all fish were sampled from a single tank (T0 and T10 from the same tank) to avoid the stress induced by fish manipulation from one sampling to the following one. Blood was collected by caudal puncture with ammonium-heparinised syringes. Plasma samples were obtained and processed as described above. Fish were killed rapidly by decapitation and liver and muscle were removed, weighed, frozen in dry ice and stored at −80°C until assayed.
Assessment of metabolite levels and enzyme activities
Plasma glucose levels were quantified by using a commercial kit (Biomérieux) adapted to a microplate format. Metabolite levels were assessed in 75 mg of liver and 150 mg of muscle homogenised immediately by ultrasonic disruption with 5·5 vols (liver) or 3·5 vols (muscle) of ice-cooled 0·6 m-perchloric acid, and neutralised (using 1 m-potassium bicarbonate). The homogenate was centrifuged (10 000 g , 4 min), and the resulting supernatant was immediately frozen in dry ice and stored at −80°C until analysis. Liver and muscle glycogen levels were assessed using the method of Keppler & Decker( Reference Keppler and Decker 37 ), and glucose obtained after glycogen breakdown (after subtracting free glucose levels) was determined with a commercial kit (Biomérieux). TAG levels in muscle and NEFA levels in plasma and liver were also analysed with commercial kits (Spinreact and Wako, respectively).
The activities of enzymes involved in glycolysis (GK and PK), glycogen metabolism (glycogen synthase (GSase) and GPase), gluconeogenesis (glucose-6-phosphatase (G6Pase), fructose-1,6-biphosphatase (FBPase) and phosphoenolpyruvate carboxykinase (PEPCK)) and lipogenesis (glucose-6-phosphate dehydrogenase (G6PDH), ATP citrate lyase (ACLY)) were determined in the various dietary treatments. Enzyme activities were assessed in 150 mg of liver and 250 mg of muscle samples homogenised by ultrasonic disruption with 7 vols (liver) or 4 vols (muscle) of ice-cold buffer consisting of 50 mm-Tris (pH 7·6), 5 mm-EDTA, 2 mm-1,4-dithiothreitol and a protease inhibitor cocktail (Sigma Chemical Co.). The homogenate was centrifuged (900 g , 10 min) and the supernatant was immediately frozen on dry ice and stored at −80°C until analysis. Enzyme activities were determined in a microplate reader SPECTRAFluor (Tecan). Reaction rates of enzymes were determined by the increase or decrease in absorbance of NAD(P)H at 340 nm. The reactions were started by the addition of homogenates (10–15 µl), at a pre-established protein concentration, omitting the substrate in control wells (final volume 265–295 µl), and allowing the reactions to proceed at 37°C for pre-established times (5–45 min). Enzymatic analyses were carried out at maximum rates, with the reaction mixtures set up in preliminary tests to render optimal activities by adapting to Senegalese sole methods previously described for rainbow trout( Reference Conde-Sieira, Soengas and Valente 31 , Reference Polakof, Míguez and Soengas 36 ). An unit of enzyme activity (U) is defined as the amount of enzyme that catalysed the hydrolysis of 1 µmol of substrate/min, and the activity was normalised by milligrams of soluble protein. Protein was assayed in triplicate in homogenates using microplates according to the bicinchoninic acid method( Reference Smith, Krohn and Hermanson 38 ) using bovine serum albumin (Sigma) as standard.
Statistical analysis
All results are expressed as mean values with their standard errors. Statistical differences were assessed with a three-way ANOVA, with diet (48:38, 52:34, 56:30 or 60:26), time (0, 1, 5 and 10 h) and phase (initial and final) as main factors. Only in those cases in which a significant effect was noted within a factor, post hoc comparisons were carried out by a Student–Newman–Keuls test, and differences were considered statistically significant at P<0·05. When necessary, data were log-transformed to fulfil the conditions of the ANOVA. In the case of growth parameters and feed intake, statistical analyses were performed by one-way ANOVA with dietary treatment as the main factor.
Results
Fish mortality was ≤1 % in all experimental treatments. All groups exhibited around a 2·5-fold increase in their initial body weight (22·0 g) after the 15-week experimental period (Table 2). No significant differences related to DGI were observed among dietary treatments (range of 0·9–1·0). The Hepatosomatic Index (HSI) was also similar among fish (0·9–1·1). A significant decrease in FCR values was observed in sole fed diets with increasing protein (values ranging from 1·5 to 1·2 in 48:38 and 60:26 diets, respectively). VFI decreased significantly when dietary CH was progressively substituted by protein. As a consequence, lipid, CH and energy intake followed the same trend. The lowest DM and energy retention was obtained for fish fed the 48:38 diet, as a result of increased DM intake. Sole fed the 52:34 diet showed significantly higher protein retention than those fed the 48:38 diet (Table 2).
Table 2 Growth performance parameters and feed intake of Senegalese sole fed the experimental diets for 104 d (Mean values with their standard errors; n 3)
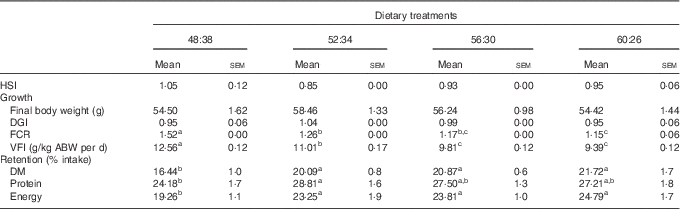
HSI, Hepatosomatic Index: liver weight (g)/body weight (g); DGI, Daily Growth Index: 100×((W1)1/3−(W0)1/3)/d, where W0 and W1 are the initial and the final fish mean weights (g); FCR, feed conversion ratio: dry food intake (g)/weight gain (g); VFI, voluntary feed intake: cumulative dry feed consumption/average body weight (ABW) per d, where ABW (kg) was (W1+W0)/2.
a,b,c Mean values with unlike superscript letters were significantly different (P<0·05) between dietary conditions.
The statistical significance of the differences observed in GTT and the metabolic parameters assessed in plasma, liver and muscle, attributed to main factors in three-way ANOVA, are shown in Table 3. The significant differences resulting from post hoc comparisons are detailed in each figure (Figs. 2–8). Significant differences of Fig. 1 are detailed in an annexed table in the online Supplementary material.

Fig. 1 Glucose levels in plasma after oral administration of 10 ml/kg of saline solution with glucose (1 g/kg) sampled 0, 1, 5 and 10 h after administration performed at the initial (4 d) or final (104 d) phase of the trial with four different protein:carbohydrate ratio diets (![]() , 48:38;
, 48:38; ![]() , 52:34;
, 52:34; ![]() , 56:30 or
, 56:30 or ![]() , 60:26). Values are means (n 8 fish per diet and postprandial time), with standard errors. Statistical differences (P<0·05) are indicated in the annexed tables in the online Supplementary material. GTT, glucose tolerance test.
, 60:26). Values are means (n 8 fish per diet and postprandial time), with standard errors. Statistical differences (P<0·05) are indicated in the annexed tables in the online Supplementary material. GTT, glucose tolerance test.
Table 3 P-values obtained after three-way ANOVA of parameters assessed in Senegalese sole. Diet (48:38, 52:34, 56:30 or 60:26), time (0, 1, 5, and 10 h) and phase (initial and final) were the main factors. Diet×time, diet×phase and time×phase are the first-order interactions. Diet×time×phase is the second-order interactionFootnote *

GK, glucokinase; PK, pyruvate kinase; GPase, glycogen phosphorylase; GSase, glycogen synthase; FBPase, fructose-1,6-biphosphatase; G6Pase, glucose-6-phosphatase; PEPCK, phosphoenolpyruvate carboxykinase; G6PDH, glucose-6-phosphate dehydrogenase; ACLY, ATP citrate lyase; HK, hexokinase.
* All values are significantly different unless noted by a dash.
GTT results (Fig. 1) indicate no changes among diets at the initial phase of the experiment. Increments of plasma glucose levels were observed at T1 and T5, although they were not significantly different from basal levels. These increased levels were totally recovered at 10 h in all dietary groups. At the end of the experiment, GTT also showed higher increments of glucose at T1 and T5 with total recovery of basal levels at T10, except in fish fed the 60:26 diet that had significantly higher values than those fed 52:34 and 48:38 diets.
As for metabolite levels in plasma (Fig. 2), increments of glucose levels at the initial phase of the trial were observed 1 and 10 h after feeding diets with high CH content (48:38 and 52:34). At the end of the experiment, postprandial increments of plasma glucose levels were observed in all groups except 56:30, and, in general, glucose levels were lower at this phase than at the beginning. Values obtained for plasma NEFA levels tended to decrease postprandially (more clearly in 48:38 and 56:30), whereas increased levels were observed in 60:26 at the final phase.

Fig. 2 Glucose and NEFA levels in plasma at different postprandial times (0, 1, 5 or 10 h) after feeding four experimental diets (![]() , 48:38;
, 48:38; ![]() , 52:34;
, 52:34; ![]() , 56:30 or
, 56:30 or ![]() , 60:26) with different protein:carbohydrate ratios during 4 d (initial) or 104 d (final). Values are means (n 8 fish per diet and postprandial time), with standard errors. a,b Mean values within a column with unlike letters were significantly different (P<0·05) between dietary treatments. Symbols indicate differences (P<0·05) between postprandial times: * different from T0; † different from T1; ‡ different from T5 and § different from T10.
, 60:26) with different protein:carbohydrate ratios during 4 d (initial) or 104 d (final). Values are means (n 8 fish per diet and postprandial time), with standard errors. a,b Mean values within a column with unlike letters were significantly different (P<0·05) between dietary treatments. Symbols indicate differences (P<0·05) between postprandial times: * different from T0; † different from T1; ‡ different from T5 and § different from T10.
In liver, glycogen values (Fig. 3) were in general higher in fish fed CH-enriched diets at the initial phase of the experiment, whereas at the end of the experiment glycogen levels did not respond to dietary treatment and showed lower values than at the initial phase. Postprandial NEFA levels tended to increase after feeding low protein:CH diets and to decrease in fish fed with high protein:CH diets. Thus, lower NEFA levels were observed in fish fed the 48:38 diet than those fed the 60:26 diet, both at T0 and T5, whereas the opposite was observed at T10. At the final phase of the experiment, levels of hepatic NEFA were lower than in the beginning.

Fig. 3 Glucose, glycogen and NEFA levels in liver at different postprandial times (0, 1, 5 or 10 h) after feeding four experimental diets (![]() , 48:38;
, 48:38; ![]() , 52:34;
, 52:34; ![]() , 56:30 or
, 56:30 or ![]() , 60:26) with different protein:carbohydrate ratios during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.
, 60:26) with different protein:carbohydrate ratios during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.
An increased postprandial glycolitic potential indicated by GK and PK activities in liver (Fig. 4) was observed in fish fed all diets at the beginning of the experiment. In the final phase, increased postprandial values were observed in fish fed CH-enriched diets at different times: the 48:38 group presented higher GK activity at T10 than at T0, whereas the 52:34 group showed higher GK activity at T1 than at T0 and T10; PK activity was also higher at T5 compared with T0 in the 48:38 dietary group. However, these postprandial increments were not observed in fish fed a lower dietary CH content (56:30 and 60:26). Moreover, basal levels (T0) in these dietary groups (56:30 and 60:26) were higher than those presented by diets with a high CH content (48:38). Postprandial GPase activity decreased in the 48:38 diet compared with basal levels at T5 and T10 and GSase responded to CH content in 48:38 and 60:26 diets at the final but not at the initial phase of the experiment (Fig. 4). The postprandial gluconeogenic activity in liver (Fig. 5), indicated by the activities of FBPase, G6Pase and PEPCK, tended to decrease in the 48:38 group at the beginning of the experiment, but after 104 d of treatment an increased gluconeogenic potential was observed. In general, lower values of G6Pase activity were observed at the final phase of the experiment compared with the initial levels. Enzymes related to the lipogenic pathway, such as G6PDH, showed higher activity in 48:38 than 60:26 dietary treatment; the opposite trend was observed for ACLY, which in addition presented lower values at the final than at the initial phase of the experiment. However, G6PDH activity tended to be higher at the final than at the initial phase of the experiment (Fig. 6).

Fig. 4 Enzyme activity (mU/mg protein) related to glycolisis and glycogen metabolism in liver. Glucokinase (GK), pyruvate kinase (PK), glycogen phosphorylase (GPase) and glycogen synthase (GSase) activity at different posprandial times (0, 1, 5 or 10 h) after feeding four experimental diets (![]() , 48:38;
, 48:38; ![]() , 52:34;
, 52:34; ![]() , 56:30 or
, 56:30 or ![]() , 60:26) with different protein:carbohydrate ratios during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.
, 60:26) with different protein:carbohydrate ratios during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.

Fig. 5 Enzyme activity (mU/mg protein) related to glucoenogenesis in the liver. Fructose-1,6-biphosphatase (FBPase), glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK) activity at different postprandial times (0, 1, 5 or 10 h) after feeding four experimental diets (![]() , 48:38;
, 48:38; ![]() , 52:34;
, 52:34; ![]() , 56:30 or
, 56:30 or ![]() , 60:26) with different protein:carbohydarte ratios during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.
, 60:26) with different protein:carbohydarte ratios during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.

Fig. 6 Enzyme activity (mU/mg protein) related to lipogenesis in liver. Glucose-6-phosphate dehydrogenase (G6PDH) and ATP citrate lyase (ACLY) activity at different postprandial times (0, 1, 5 or 10 h) after feeding four experimental diets (![]() , 48:38;
, 48:38; ![]() , 52:34;
, 52:34; ![]() , 56:30 or
, 56:30 or ![]() , 60:26) with different protein:CH ratios during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.
, 60:26) with different protein:CH ratios during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.
In muscle, increases in glucose levels were observed in the different dietary groups at different postprandial times at the initial phase of the experiment, but higher glucose and glycogen levels were obtained in fish fed diets with less CH content in the final phase (Fig. 7). TAG levels showed postprandial increments in 48:38 and 56:30 dietary groups at the initial, but not at the final, phase. Moreover, TAG values were generally lower at the final phase than at the initial one. An increased postprandial glycolitic potential (indicated by HK activity), as well as higher GSase activity, was observed specially in groups fed CH-enriched diets for an extended time (Fig. 8).

Fig. 7 Glucose, glycogen and trygliceride levels in muscle at different postprandial times (0, 1, 5 or 10 h) after feeding four experimental diets (![]() , 48:38;
, 48:38; ![]() , 52:34;
, 52:34; ![]() , 56:30 or
, 56:30 or ![]() , 60:26) with different protein:carbohydrate ratio during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.
, 60:26) with different protein:carbohydrate ratio during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.

Fig. 8 Enzyme activity (mU/mg protein) of hexokinase (HK) and glycogen synthase (GSase) in muscle at different postprandial times (0, 1, 5 or 10 h) after feeding four experimental diets (![]() , 48:38;
, 48:38; ![]() , 52:34;
, 52:34; ![]() , 56:30 or
, 56:30 or ![]() , 60:26) with different protein:carbohydrate ratios during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.
, 60:26) with different protein:carbohydrate ratios during 4 d (initial) or 104 d (final). Further details are indicated in Fig. 2 legend.
Discussion
The use of CH in aquafeeds is of increased interest, as the replacement of marine protein sources by vegetable ones and the reduction of protein use as the main ingredient are desirable goals in current aquaculture( Reference Stone 2 , Reference Cabral, Bacelar and Batista 39 ). However, fish present divergent capacity to utilise dietary CH, and in some cases, especially in carnivorous species, this capacity is not adequate because of anomalies in glucose metabolism( Reference Polakof, Panserat and Soengas 3 ). Senegalese sole seems to possess a good ability to deal with glucose loads( Reference Conde-Sieira, Soengas and Valente 31 ), but this capacity can be influenced by the presence of other dietary macronutrients such as lipids( Reference Borges, Valente and Véron 16 ). In the present study, feeding Senegalese sole with low-fat diets of increased protein:CH ratio had no effect on final body weight and daily growth rate, confirming previous studies in this species( Reference Guerreiro, Peres and Castro 32 , Reference Dias, Rueda-Jasso and Panserat 40 ). In contrast, other carnivorous fish species such as rainbow trout, brown trout (Salmo trutta) and Atlantic salmon showed reduced growth with the use of low protein:CH ratios in diet( Reference Krogdahl, Sundby and Olli 11 , Reference Hillestad, Johnsen and Åsgård 41 , Reference Viaplana-Marín, Fernández-Borrás and Blasco 42 ). Furthermore, all fish had similar HSI, and no deleterious healthy effects were detected in the present study, even considering the extended experimental time (104 d) and the elevated content of CH used on the tested diets (up to 38 % DM). This is in contrast with previous studies in other carnivorous fish species in which high HSI levels or liver hypertrophy were observed after using elevated contents of CH in the diet( Reference Rawles, Smith and Gatlin 43 ).
VFI of fish was significantly increased when dietary protein was progressively substituted by CH, confirming the strong dependency of this species on protein sources to display maximal growth( Reference Rema, Conceição and Evers 27 ). However, the 48:38 dietary group showed the lowest values of DM, energy and protein retention, which indicates that the highest VFI presented in this group respond to an essential protein requirement for energy purposes rather than for tissue accretion. No differences were found among the other dietary groups regarding nutrient and energy retention, suggesting that a protein:CH ratio of 52:34 is enough to induce maximal growth. The effect of the diets used in the present study on nutrient utilisation and flesh quality will be further discussed elsewhere (Salas-Leiton et al., unpublished results).
To see whether the use of diets with different contents of CH during an extended period could have some influence on glucose tolerance in Senegalese sole, GTT were performed at the initial (4 d) and final phases (104 d) of the experiment. An oral GTT is commonly used to evaluate the capacity to restore glucose homoeostasis after an induced hyperglycaemia, and this has been carried out in many fish species( Reference Moon 44 ). The results are very divergent depending on the fish species, but, in general, omnivorous/herbivorous fish show better capacity to tolerate glucose than carnivorous species( Reference Polakof, Panserat and Soengas 3 ). In a previous study, Conde-Sieira et al.( Reference Conde-Sieira, Soengas and Valente 31 ) suggested that Senegalese sole, despite its carnivorous feeding habits, have a high capacity to deal with glucose loads and present fast glucose clearance rates comparable to those of omnivorous and/or herbivorous species. However, that study was carried out in fish fed a commercial diet (62 % crude protein, 16 % lipids and 9 % CH) and did not allow predicting whether such a better glucose tolerance could be sustained in fish fed CH-enriched diets over long-term periods. In the present study, the initial GTT was quite close to that observed before( Reference Conde-Sieira, Soengas and Valente 31 ), with glucose levels reaching the maximum 1 h after the glucose load, which again resembles the GTT described in omnivorous/herbivorous( Reference Stone 2 , Reference Furuichi and Yone 45 , Reference Lin, Ho and Shiau 46 ) species rather than that reported in other carnivorous species( Reference Garcia-Riera and Hemre 47 , Reference Hemre, Torrissen and Krogdahl 48 ). In general, fish fed with the four experimental diets succeeded in returning glycaemia to normality in between 5 and 10 h post glucose load. These results suggest an effective capacity of Senegalese sole to deal with increased dietary CH. If the initial GTT is compared with that observed at the end of the trial, two interesting observations arise: the first one is that glycaemia remained at high levels for a longer time period at the end of the trial, in a way that after 5 h of glucose load levels were basically the same as those observed 1 h after load, irrespectively of the dietary CH level; the second observation is that glycaemia in fish fed for 104 d with CH-enriched diets was recovered 10 h after glucose load (except 60:26 diet) – that is, a time period perfectly comparable to that observed at the beginning of the trial. The experimental diets used in the present study are isolipidic and with low fat content (6 %), which indicates that differences in GTT presented in 60:26 can be related to the high protein or the low dietary CH content. In this way, the deleterious effect of a high-protein diet on glucose metabolism by promoting insulin resistance or impaired gluconeogenesis regulation has been reported in mammals and fish( Reference Tremblay, Lavigne and Jacques 49 , Reference Kirchner, Kaushik and Panserat 50 ). These changes in GTT suggest that Senegalese sole has an effective tolerance to the presence of increased amounts of CH in the diet, despite a slightly reduced ability to reduce glycaemia levels in a faster way after 104 d of feeding CH-enriched diets.
The sustained glucose tolerance observed at the end of the trial is further supported by the results observed in plasma glucose levels after feeding experimental diets that, in general, were lower at the end than at the beginning of the trial. This is possible because of an improved metabolism in tissues that capture the circulating glucose more efficiently, therefore reducing glycaemia. As expected, plasma glucose values were higher in the fish fed CH-enriched diets (48:38 and 52:34), except 5 h after feeding probably because at this time glucose is being used for metabolic purposes. However, plasma glucose level did not differ significantly from basal levels, which were higher than those previously observed in Senegalese sole( Reference Conde-Sieira, Soengas and Valente 31 ). The process of glucose clearance in plasma after feeding CH is slower than that registered in the GTT, and after 10 h fish fed the 52:34 diet still had a higher glycaemia than those fed the 60:26 diet. This is probably because of a more gradual influx of glucose to the bloodstream in accordance with the digestive processes. Furthermore, the partial effects of the presence of an increased amount of CH observed at the beginning of the trial (where plasma glucose levels are higher in CH-enriched diets) basically disappeared at the end, further supporting the enhanced tolerance of CH in the diet that is not reflected in changes in glycaemia related to such CH dietary content. The higher levels of plasma glucose observed at the beginning of the experiment could be also a consequence of the nutritional background of the experimental fish, as they were previously fed a fattier diet (16 % lipids; 62 % crude protein). In this sense, it has been reported in Senegalese sole that diets containing high level of lipids could impair glucose tolerance through the down-regulation of insulin signalling pathways( Reference Borges, Valente and Véron 16 ).
Liver has a key role in maintaining glucose homoeostasis in fish submitted to different nutritional conditions( Reference Enes, Panserat and Kaushik 17 ). In the present study, the postprandial changes in hepatic levels of metabolites and enzyme activities related to pathways involved in glucose storage or production were evaluated in fish fed diets with different protein:CH contents. In general terms, glucose levels in liver did not differ among diets or time at the initial phase of the experiment, although a decrease in 56:30 diet was observed 5 h after feeding. Similarly, Conde-Sieira et al.( Reference Conde-Sieira, Soengas and Valente 31 ) have previously reported no significant changes in hepatic glucose levels in hyperglycaemic Senegalese sole after glucose administration, probably because of a fast removal of glucose in the liver.
The reasonable glucose tolerance displayed by Senegalese sole after 104 d of feeding CH-enriched diets was also reflected in the levels of glycogen in liver, which were generally lower at the end than at the beginning of the trial. Moreover, the high glycogen levels induced by the high dietary CH content at the beginning of the trial disappeared at the end of the trial. Thus, it seems that Senegalese sole is able to deal with increasing amounts of dietary CH in an effective way, avoiding its excessive accumulation as glycogen. These results point towards an effective and fast glycogen metabolism in liver, reflecting a good adaptation of Senegalese sole to increased CH in the diet. This is supported by the increased activity of enzymes involved in glycogen metabolism (particularly GPase) at the end of the feeding period compared with the beginning of the trial.
The decrease of glucose levels in plasma at the end of the trial was not because of increased levels of glycogen in the liver pointing towards an effective use of glucose as fuel through the glycolytic pathway. GK is one of the main enzymes implicated in this pathway, and it has been reported to be nutritionally induced in many fish species( Reference Enes, Panserat and Kaushik 17 , Reference Guerreiro, Peres and Castro 32 ) including Senegalese sole( Reference Conde-Sieira, Soengas and Valente 31 ). Accordingly, significant increments of GK activity were observed 10 h after feeding in all diets, with the exception of fish fed the lowest dietary CH content (60:26). This enhancement was of the same order of magnitude regardless of the dietary CH level (except 60:26), which may be because of a possible inhibition of GK expression when plasma glucose levels are elevated, as previously observed in rainbow trout( Reference Skiba-Cassy, Panserat and Larquier 51 ). GK activities were in general higher at the end of the trial than at the beginning, suggesting a better capacity of phosphorylating glucose, which might explain the better postprandial glycaemic control observed at this stage. Similar results were previously reported in omnivorous (but not carnivorous) fish species( Reference Capilla, Medale and Panserat 21 ). However, PK, another enzyme involved in glycolisis, but whose activity is known to be poorly induced by nutritional conditions in fish( Reference Enes, Panserat and Kaushik 17 ), was not correlated to dietary CH content. In fact, the diet with a higher CH level showed the lowest PK activity 10 h after feeding. The possible activation of the glycolytic capacity at the end of the trial might have coincided with an inhibition of the gluconeogenic potential resulting in a decreased liver capacity to export glucose into plasma. This capacity can indeed be assessed by changes in the activity of G6Pase, which showed a general decrease in the activity at the end of the trial v. the beginning. However, when the other two gluconeogenic enzymes (FBPase and PEPCK) were assessed, the expected inhibition was not observed. Previous studies in Senegalese sole also reported an inhibition of endogenous glucose production after glucose administration or, similar to the present study, after feeding high levels of CH( Reference Conde-Sieira, Soengas and Valente 31 , Reference Guerreiro, Peres and Castro 32 ). These results reinforce the idea that Senegalese sole posses a good capacity to deal with glucose loads. However, the postprandial enzyme activities observed at the end of the experiment indicate no inhibition of gluconeogenesis. This lack of gluconeogenesis inhibition can be because of the influence of other macronutrients present in the diet, that is protein. There is increasing evidence that amino acids interfere with insulin function, affecting glucose homoeostasis by promoting insulin resistance and increasing gluconeogenesis( Reference Tremblay, Lavigne and Jacques 49 ). Recent studies reported that high dietary CH per se are unable to inhibit G6Pase activity( Reference Panserat, Capilla and Gutierrez 14 , Reference Enes, Panserat and Kaushik 17 , Reference Enes, Panserat and Kaushik 19 ), but dietary amino acids intake could also have a role and be a limiting factor for endogenous hepatic glucose production( Reference Kirchner, Seixas and Kaushik 20 , Reference Kirchner, Kaushik and Panserat 50 ).
The changes observed in lipid metabolism are indirectly related to the changes observed in glucose metabolism. If a fish is not able to effectively deal with increased levels of dietary CH, this could result in increased deposition of lipids through up-regulation of de novo lipogenesis. In fact, many lipogenic enzymes were shown to be induced by a high-CH diet in mammals( Reference Towle, Kaytor and Shih 52 ). In fish fed CH-enriched diets, an up-regulation of lipogenesis was observed in coho salmon( Reference Lin, Romsos and Tack 53 ) but not in rainbow trout( Reference Panserat, Skiba-Cassy and Seiliez 23 ). Postprandial stimulation of lipogenic enzymes, such as ACLY, was also observed in rainbow trout refed a high-protein diet( Reference Seiliez, Panserat and Lansard 54 ). In Senegalese sole feeding diets with high CH and low lipid content also seems to promote hepatic lipogenesis( Reference Guerreiro, Peres and Castro 32 , Reference Dias, Rueda-Jasso and Panserat 40 ), although no changes in G6PDH activity were observed( Reference Conde-Sieira, Soengas and Valente 31 , Reference Guerreiro, Peres and Castro 32 ). In the present study, and at the end of the trial, levels of NEFA in plasma were in general lower than those observed at the beginning of the trial. This suggests that CH are being effectively metabolised. In fact, in the liver, NEFA levels were also generally lower in fish at the end than at the beginning of the trial, suggesting a reduced lipogenic capacity that is further supported by the concomitant decrease in ACLY activity towards the end of the trial. Furthermore, in general, the higher the CH levels in the diet, the lower the ACLY activity, especially at the first postprandial hours. The absence of marked changes in liver lipogenic potential strengthens the important glucose tolerance of Senegalese sole, as the extra available glucose resulting from feeding CH-enriched diets is used and not re-directed to synthesise lipid. However, considering that amino acids can be also used for lipogenesis, we cannot discard that the interchange of glucose with amino acids would explain the maintenance of lipogenesis in the liver.
The capacity of Senegalese sole to use CH as an energy source in the muscle is not as clear as in the liver. Non-carnivorous fish species have demonstrated a higher capacity to phosphorylate glucose in muscle compared with carnivorous ones( Reference Polakof, Panserat and Soengas 3 , Reference Capilla, Medale and Panserat 21 ). However, dietary CH seem unable to induce significant changes in glucose metabolism in carnivorous fish muscle, which has been attributed to their glucose intolerance( Reference Hemre, Mommsen and Krogdahl 1 , Reference Panserat, Skiba-Cassy and Seiliez 23 ). In the present study, a decreased capacity of muscle to use glucose through glycolysis was observed at the final phase of the experiment compared with the initial one. This could be related to the simultaneous enhanced use of glucose noted in liver and to the fact that this species is more tolerant to glucose than other carnivorous fish species. However, this not reflected in changes in glucose or glycogen levels in the muscle, although increased GSase activity was noted. TAG levels in muscle displayed lower levels at the final than at the initial phase of the experiment, which is in accordance with the lower lipogenic potential also observed in the liver. This could also be because of the lower lipid content of the experimental diets compared with those fed before the feeding trial, which are commonly used in commercial conditions (6 v. 16 % fat).
In summary, the results obtained in this study clearly suggest a good glucose tolerance in Senegalese sole and provide valuable information regarding the impact of feeding CH-enriched diets for a long-term period on its metabolism. Senegalese sole tolerated important amounts of CH in the diet without showing any deleterious signs in terms of growth or any metabolic disorders. Moreover, the control of glycaemia was maintained after 104 d of feeding diets with an important amount of CH, and, in general, postprandial glucose levels in plasma were even lower than at the beginning of the experiment. Importantly, fish metabolic response after 104 d of feeding was basically the same, irrespective of the dietary amounts of CH that were used. This indicates that the enzymatic machinery related to glucose metabolism was working efficiently in order to compensate the glucose load generated by CH-enriched diets, reflecting a reasonable tolerance of Senegalese sole to such diets. This tolerance to glucose is also reflected by an increased use of glucose through glycolysis in liver, as well as by the non-necessity of increasing the lipogenic potential in the same tissue. In conclusion, the present results indicate that Senegalese sole seems to deal reasonably well with diets rich in CH. However, this enhanced capacity to use CH implies a higher energy investment because of an elevated feeding activity in order to satisfy the high protein requirements of this species. Further studies where protein supply should be limited would help to clarify whether a protein-sparing effect of dietary CH is possible in Senegalese sole under a fixed ration of food supplied.
Acknowledgements
This work was partially supported by NORTE-07-0124-FEDER-000038, in the context of the North Regional Operational Programme (ON.2 – O Novo Norte), under the project Sustainable Aquaculture and Animal Welfare (AQUAIMPROV) to L. M. P. V., and from Spanish Ministerio de Economía y Competitividad and European Fund for Regional Development (AGL2013-46448-3-1-R and FEDER) to J. L. S. M. C.-S. was recipient of a postdoctoral fellowship from Fundação para a Ciência e a Tecnologia (FCT) (SFRH/BPD/84251/2012) and from Xunta de Galicia (Plan I2C). E. S.-L. was partially covered by Andalusian Operational Program-European Social Fund (2007–2013), axis III.
M. C.-S., L. M. P. V. and J. L. S. conceived and designed the experiments; M. C.-S., E. S.-L. and N. F. P. performed the experiments; M. C.-S., E. S.-L., N. F. P. and M. M. D. analysed the data and M. C.-S., L. M. P. V. and J. L. S. wrote the paper.
There are no conflicts of interest in connection with the present study.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516001057
















