Article contents
Experimental investigation of phase equilibria in the Ni–Fe–Zr ternary system
Published online by Cambridge University Press: 25 April 2016
Abstract
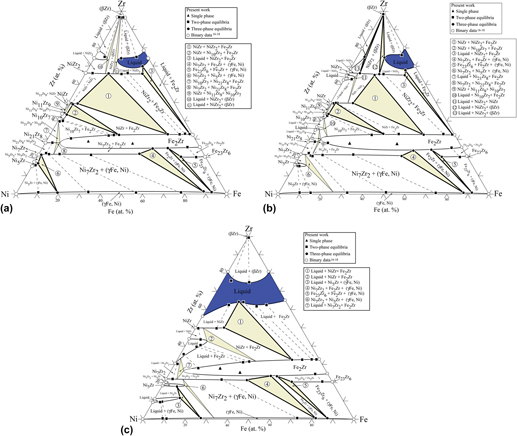
Three isothermal sections of the Ni–Fe–Zr ternary system at 1000, 1100, and 1200 °C were experimentally determined using equilibrated ternary alloys. No ternary compound is found in this system. The obtained experimental results show that among three isothermal sections, the (γFe, Ni) phase region extends from the Ni-rich corner to the Fe-rich corner, and the solubility of Zr in the (γFe, Ni) phase is small. The phase equilibrium at 1100 °C is similar to that at 1000 °C. The Ni5Zr, Ni10Zr7, and Fe2Zr phases have solid solution composition ranges, but the Ni7Zr2, Ni21Zr8, NiZr, NiZr2, and Fe23Zr6 phases almost exhibit nearly linear compounds both at 1000 and 1100 °C. The solubilities of Fe in Ni7Zr2 phase and Ni in Fe2Zr phase are extremely large. At 1200 °C, the liquid phase of Zr-rich corner forms the continuous region from the Ni–Zr side to the Fe–Zr side. Additionally, the solubilities of Fe in Ni5Zr, NiZr phases and Ni in Fe23Zr6 phase clearly increase with increasing temperature to 1200 °C. The obtained results may provide a better understanding of microstructures and further development of the Ni–Fe–Zr alloys.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 4
- Cited by





