The understanding of the pathophysiology of TTTS is relatively recent and still incomplete due to the lack of experimental models but also because the disease is strictly limited to intrauterine life (Blickstein, Reference Blickstein1990). Prenatal diagnosis of chorionicity by ultrasound has been key to timely recognition of severe forms of TTTS (Sebire et al., Reference Sebire, Snijders, Hughes, Sepulveda and Nicolaides1997). Management rather than treatment has remained symptomatic for over 20 years, mainly through serial amnioreduction, which attempts to delay delivery up to an acceptable prematurity for the twins to be delivered (Mari et al., Reference Mari, Roberts, Detti, Kovanci, Stefos, Bahado-Singh and Fisk2001). The critical role of inter-twin anastomoses running on the chorionic plate had long been recognized in causing specific exsanguination-related twins’ mortality and morbidity in the survivors (Bejar et al., Reference Bejar, Vigliocco, Gramajo, Solana, Benirschke, Berry and Resnik1990). However, placental surgery in TTTS could only become available with the development of medical laser technology and the miniaturization of operative endoscopes in the early 1990s (De Lia et al., Reference De Lia, Kuhlmann, Harstad and Cruikshank1995; DeVore et al., Reference DeVore, Dixon and Hobbins1983; Ville et al., Reference Ville, Hyett, Hecher and Nicolaides1995). Equipoise on the efficacy of laser surgery among specialists in fetal medicine was established over 10 years, and a randomized trial comparing serial amnioreduction to endoscopic laser surgery was both timely and conclusive (Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). Intrauterine endoscopic placental surgery in TTTS is the first procedure of fetal therapy with a benefit established through a successful randomized control trial (RCT; Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). In addition, this procedure has become a widely accepted first-line treatment in TTTS following prenatal diagnosis. The reasons for success are many: (1) the prognosis of the severe forms of the condition was dismal and yet damage was both functional and progressive in otherwise normal fetuses; (2) The procedure could be performed percutaneously and under local anesthesia, therefore bearing a high acceptability by pregnant women; (3) Diffusion, reproducibility, and refinements of the technique were achieved over a few years both through direct mentorship by founding centers within the Eurofetus consortium (Morris et al., Reference Morris, Selman, Harbidge, Martin and Kilby2010) and the sharing of experience between centers through annual meetings (European Commission, n.d.) and a prolific literature (Roberts et al., Reference Roberts, Neilson, Kilby and Gates2014). The latest completed randomized trial suggested that technical adjustment could further decrease the rate of post-operative complications, although survival was not affected (Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014).
Since survival of at least one twin is around 80–85% following laser surgery in TTTS, clinically meaningful changes in survival are unlikely to show in randomized studies. In this article, a post-hoc analysis of the Eurofetus trial was conducted using a Bayesian methodology to investigate the credibility of this trial under different hypotheses. We will also review the rationale for another RCT that is currently being conducted in stage 1 TTTS cases (Quintero et al., Reference Quintero, Morales, Allen, Bornick, Johnson and Kruger1999; U.S. National Institutes of Health, n.d.).
Revisiting the Eurofetus Trial With Bayes
The purpose of such an analysis is to put the results of the trial in perspective, based upon available knowledge from published data and archetypal prior scepticism regarding the true benefit of laser surgery compared to amnioreduction in severe TTTS. The Bayesian approach allows us to incorporate prior information as a probability distribution into the raw data available from the trial (the likelihood) and to derive a posterior distribution of the effect of treatment. This posterior distribution determines a credibility interval for the measure of association between the outcome and the treatment option. Bayesian terminology refers to credibility intervals rather than confidence intervals, but their interpretation is quite similar in practice.
We used the odds ratio (OR) as the measure of association for treatment effect and considered the asymptotical Gaussian approximation of the log odds for the analysis. Under Gaussian priors, the posterior distribution of the log odds was derived from the conjugate-normal distribution and presented as 95% credibility intervals.
Several priors were considered:
-
1. An archetypal sceptical prior (Freedman et al., Reference Freedman, Spiegelhalter and Parmar1994; Higgins & Spiegelhalter, Reference Higgins and Spiegelhalter2002; Spiegelhalter et al., Reference Spiegelhalter, Freedman and Parmar1994) regarding the treatment effect that was translated as a normal distribution of the log odds with a mean = 0 and a standard deviation = 0.35. This is equivalent to an OR = 1 with 95% CI [0.5–2]. This sceptical prior neglects any published data.
-
2. A critically sceptical prior, which is the prior distribution of mean = 0 (no difference between treatments) but with a variance sufficiently small to make the posterior 95% credibility interval reach the zero effect (OR = 1) line, thus making the posterior result of the trial inconclusive. This is equivalent to finding an archetypal person sufficiently convinced of the absence of treatment effect that his opinion remains unchanged even after the data from the trial was made public.
-
3. A historical prior incorporating the published data so far. This prior was based upon the two retrospective studies available at the time of the trial comparing serial amniodrainage and percutaneous laser surgery (Hecher et al., Reference Hecher, Plath, Bregenzer, Hansmann and Hackelöer1999; Quintero et al., Reference Quintero, Dickinson, Morales, Bornick, Bermúdez, Cincotta and Allen2003). A pooled OR was derived from the published data from these two studies with a fixed effects meta-analysis and using the Mantel–Haenszel method (Table 1). Of notice, no trials comparing percutaneous laser and amnioreduction have been published since the Eurofetus trial, except for the NIH-funded trial, which we excluded because of major differences in the treatment protocol and because of the lack of statistically sound data.
-
4. Finally, the archetypal sceptic prior and the historical prior were sequentially combined, meaning that the posterior distribution of the sceptical prior combined with the historical data is used, in turn, as the prior distribution for the trial (Brophy & Joseph, Reference Brophy and Joseph1995; Greenhouse & Wasserman, Reference Greenhouse and Wasserman1995; Tan et al., Reference Tan, Machin, Tai, Foo and Tan2002). This analysis is provided along with a sensitivity analysis in which the importance of the historical data is weighed from 0 (the historical data is irrelevant, equivalent to prior 1) to 1 (the historical data has the same weight as the trial). This allows presenting the credibility of the trial for an archetypal sceptical with increasing confidence in the pre-trial published data. Hence, by giving a weight of zero to the historical data, we return to situation 1, which ignored this data.
TABLE 1 Historical Data for the Perinatal Survival of at Least One Twin
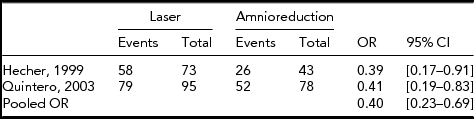
Three outcomes have been chosen for the analysis. Perinatal survival of at least one twin is the only one clearly reported in all studies and used as a main outcome in the Eurofetus trial. The Eurofetus trial also presented survival of at least one twin at 6 months, although this outcome was not available from the two historical studies. Thus, the analysis of the 6-months survival used priors 1 and 2. Similarly, the data presenting the neurological morbidity in the two historical studies was not made use of, because of differences in the measured outcomes and because we did not have access to the individual data from these studies. Nonetheless, the Bayesian analysis of this outcome from the Eurofetus trial was conducted using priors 1 and 2. The trial OR was computed using a GEE binomial model to adjust for the correlation between twins and using the robust variance of the estimate. Access to the individual data from the trial made this analysis possible.
Perinatal Survival of at Least One Twin: Scepticism and Pretrial Evidence
The Eurofetus trial yielded an OR of 0.39 [0.17–0.91] for the survival of at least one twin showing a benefit in favor of laser surgery over amnioreduction. The distribution of the OR of the trial data defines the likelihood. Considering a reasonably sceptical prior (OR = 1, [0.5–2]), the trial data failed to provide convincing evidence of a benefit of laser over amnioreduction, as demonstrated by the posterior credibility interval overlapping the value 1.
Consequently, for this outcome, the critically sceptical prior is even more tolerant than the reasonable prior and would allow a magnitude of treatment effects as large as [0.46–2.20]. This critically sceptical prior can obviously not be rejected as it is less stringent than the reasonable one. However, these priors neglect any pre-trial published data.
Two retrospective studies were available at the time of the trial (Table 1), both showing a positive and significant effect of laser on the perinatal survival of at least one twin. The pooled OR for these two studies is 0.40 [0.23–0.69], technically corresponding to a Gaussian distribution N (-0.91, 0.28²) on the log-odds’ scale. Because the historical data was added to the trial data, the posterior distribution is more peaked, with a narrower interval of the resulting posterior OR (95% credibility interval = [0.26–0.61]). However, one might question the level of evidence provided by this historical data. More specifically, the amount of weight that is given to historical retrospective evidence in each one's opinion is variable and might range from 0% to 100% for any given doctor expressing scepticism. The subjectivity of the amount of confidence that is granted to the historical data may be translated into a statistical distribution that downweighs the leverage of this data on the final credibility interval. Such an analysis is presented in Table 3, with weights of 0.2, 0.5, 0.8, and 1 expressing the spectrum of possible opinions regarding the validity of this historical data. The four priors therefore combine a reasonable sceptical with increasing confidence in the pre-trial published data. A weight of 0 would yield the same results as using a simple sceptical prior, not taking into account any published evidence and as presented previously. A power of 1 is equivalent to a reasonable sceptical that would give as much weight to the historical data as to the trial data to make his opinion. Even with a weight as low as 0.2 for the historical evidence, the resulting posterior distribution shows strong evidence in favor of laser therapy. This posterior credibility interval shifts away from 1 and grows narrower as the weight of pre-trial evidence is increased, thus increasing the overall credibility of the trial data regarding perinatal survival of at least one twin.
Survival of at Least One Twin at 6 Months
In the Eurofetus trial, the postnatal follow-up was at 6 months. This outcome is important since mortality may differ or increase with follow-up time, potentially increasing the overall treatment effect. Table 2 shows that the rate of survival of at least one twin decreased in the amnioreduction group in the time interval following the perinatal period (39/70 to 36/70), whereas it remained unchanged in the laser group (55/72). Although it is a more compelling outcome than perinatal survival, 6 months’ survival was not reported in the two historical studies. Nonetheless, the credibility of the Eurofetus trial regarding this outcome was investigated using sceptical priors. Using a reasonably sceptical prior, the trial showed a strong benefit in favor of laser surgery, with a posterior 95% credibility interval of [0.35–0.94]. The critically sceptical prior distribution is a narrower distribution, with a prior confidence interval of [0.57–1.77], indicating that in order to challenge the benefit of laser over amnioreduction one must be confident that the true treatment effect will lie within these boundaries. Although quite stringent, this prior opinion cannot be rejected straightforwardly.
TABLE 2 Eurofetus Trial: Survival of at Least One Twin and Individual Intact Survival
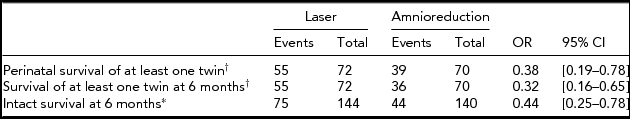
†Outcome defined at the pregnancy level (n = 72 and 70 respectively); *outcome defined at the fetal level (n = 144 and 140 respectively).
Intact Individual Survival at 6 Months
The goal of any prenatal treatment is to deliver a healthy baby. Intact individual survival at 6 months is a composite outcome that was used in the Eurofetus trial because of the concern that a treatment could independently increase the rate of neurological morbidity. Although this specific outcome had not been reported in previous studies, it appears to be the most important for comparing treatment effects as it comprises not only survival but also neurological damage at 6 months. Compared to the previous outcomes, this outcome is at the fetal level, with 144 and 140 fetuses in each group respectively, instead of pregnancy level outcomes, such as survival of at least one twin, and thus requires specific statistical handling to take into account the correlation between the twins. The credibility of the Eurofetus trial regarding this outcome was investigated using priors 1 and 2 as previously. The trial data yielded strong evidence in favor of laser treatment with an OR of 0.44 [0.25–0.78] using a GEE logistic model. Under the hypothesis of a reasonable sceptical prior (OR = 1 [0.5–2]), the trial provided strong evidence in favor of laser with a posterior credibility interval for the OR of [0.39–0.95]. The critically sceptical prior sufficiently convinced of the absence of treatment difference to invalidate the superiority of laser over amnioreduction is the distribution with an OR = 1 [0.58–1.72]. As with the previous outcome, this critical sceptical cannot be straightforwardly rejected on the basis that it is unreasonable, although it may be argued such a stringent opinion prior to the trial is unlikely.
Clinically Worthwhile Improvement
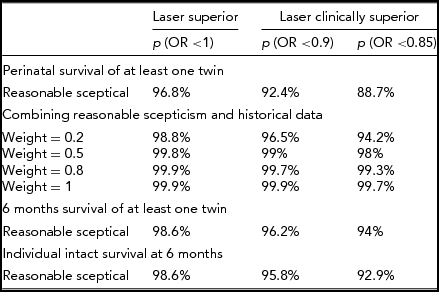
The previous analyses showed how scepticism could influence the credibility of the absolute superiority of laser over amnioreduction. However, a trial is designed and powered to detect a predefined expected difference in outcome that encompasses a clinically worthwhile improvement. This improvement represents the minimal difference δ that would impact on the decision process of changing to a new treatment. The Eurofetus trial was designed to detect a δ = 15% reduction in mortality. Table 3 shows the posterior probabilities for a reasonable sceptical and with three levels of treatment benefit. The first is the probability that the OR is inferior to 1, p (OR <1), and represents the absolute probability of superiority of laser, disregarding any clinically relevant differences. Two thresholds were chosen as clinically worthwhile differences: a 10% and a 15% improvement that translate into p (OR <.9) and p (OR <.85) respectively. For both levels of clinically relevant improvement, the trial data provided convincing credibility probability for most outcomes. However, considering perinatal survival of at least one twin and a 15% improvement, the posterior credibility probability is ‘only’ 88.7% and would not resist even a unilateral 5% significance level. Adding pretrial evidence drastically increases these probabilities. The trial provided very high credibility probabilities of a benefit of laser on intact survival at 6 months. Even considering 15% as the minimal clinically worthwhile difference would yield a 92.9% credibility of clinical superiority of laser for this outcome under a reasonably sceptic hypothesis.
TABLE 3 Summary Table of Posterior Credibility Probabilities According to Clinically Worthwhile Differences With a Reasonably Sceptical Prior
Bayesian methods allow the interrogation of the trial data in a much more straightforward way than p values (Goodman, Reference Goodman1999a; Reference Goodman1999b), integrating pragmatic individual beliefs in the appreciation of the results.
The data from Eurofetus trial has provided strong evidence of a benefit of laser over amnioreduction in severe TTTS. However, and although a significant difference was found between the treatment groups, the Data Monitoring Committee chose to stop the trial at the second interim analysis after 50% of the trial was completed. We used a Bayesian methodology to investigate how a sceptical opinion could dampen the credibility of this data. For most of the analyzed outcomes, the trial data resisted a reasonable sceptical regarding the absolute superiority of laser surgery. Nonetheless, for perinatal survival of at least one twin, we showed that the trial data failed to convince a reasonable sceptical, ignoring previous published data. However, implementing the knowledge of historical studies in this opinion changed the posterior conclusion of this archetypal sceptical regarding the conclusion of the trial. Although this historical data comprised only two retrospective studies, it is of value for drawing an overall conclusion regarding the treatment effect on perinatal survival of least one twin. The impact on the posterior conclusion of adding this data to an archetypal sceptical was addressed by a sensitivity analysis with variable weights. Regarding perinatal survival of at least one twin, this analysis showed that the trial would convince an aware archetypal sceptical, even with a low granted confidence in this pre-trial evidence. Moreover, this historical data was published by two different centers that did not subsequently participate in the Eurofetus trial.
The most appealing analyzed outcome is individual intact survival at 6 months because it encompasses both survival and neurological morbidity in a single outcome and with a longer follow-up than perinatal outcomes. Although this outcome was not used for power computation in the design of the trial, the data was sufficient to convince an archetypal sceptical of an absolute as well as a clinically relevant benefit of laser over amnioreduction.
Is another similar trial needed or is the published data sufficient for changing practice? Although the trial was stopped at a 50% completion, the difference in intact survival was important enough between the treatment groups to successfully drive an archetypal sceptical to consider laser treatment as the best treatment. The decision to stop the trial at an interim analysis because of a significant difference in a sequential design is a normal process, although it might lower the impact of the whole trial because of the smaller number of patients included in each arm. Bayesian analysis showed that despite this relatively small number (72 and 70 pregnancies in each arm, or equivalently 144 and 140 fetuses), the strength of the treatment effect resisted a reasonable sceptical. Thus, this trial in itself should be considered sufficiently convincing for most. To decide whether to change practice and surgical strategy from this single trial is a difficult question. Some would advocate at least two conclusive trials before deciding to switch to a new treatment. However, in the field of prenatal medicine, because of the rarity of the disease involved, technical issues as well as ethical issues, such a scheme is unlikely or within an unbearable time-frame. We must therefore resort to changing medical practice without the quantity of data required in other fields, such as cardiovascular medicine. So, the question becomes: Is the published data sufficiently convincing for changing practice? Here lies one of the interests of Bayesian methods. If a trial is sufficiently informative to convince almost everybody with proof beyond reasonable doubt, the basis of equipoise is rejected and the best treatment in the trial should become the standard treatment, making subsequent trials unethical. However, there are numerous reasons that warrant confirmatory trials before adopting a new treatment and the previous statement is unfairly simplistic. One of these reasons is the confirmation of a clinically worthwhile improvement. We have demonstrated that even with a clinically relevant difference as large as 15%, the trial data still resisted scepticism fairly, yielding high posterior credibility for a clinically worthwhile benefit of laser over amnioreduction. In the light of these results, a confirmatory trial with a similar design might therefore seem unnecessary, considering the ethical issues at stake and we should rather concentrate our efforts in refining the treatment strategy instead of accumulating unnecessary data.
Refining Treatment: A Randomized Trial for Stage 1
There has been growing concerns that percutaneous laser surgery may not be indicated in early or stage 1 TTTS. This idea arose from the belief that stage 1 TTTS may not warrant immediate invasive treatment and may just be followed conservatively, thus reducing the iatrogenic complications of invasive therapy in non-progressive disease, as demonstrated by small retrospective studies of early TTTS (Dickinson & Evans, Reference Dickinson and Evans2004; O'Donoghue et al., Reference O'Donoghue, Cartwright, Galea and Fisk2007; Taylor et al., Reference Taylor, Denbow, Duncan, Overton and Fisk2000). However, advocates of immediate laser surgery would argue that postponing surgery would increase the rates of spontaneous fetal demise and secondary neurological morbidity, as well as preterm premature rupture of the membranes (PPROM) and very preterm birth. Indeed, Quintero staging is not the only potential prognostic factor after laser surgery and management should also encompass gestational age at diagnosis and cervical length.
The basis of equipoise has been investigated among a panel of eight specialists from the Eurofetus program using an anonymous internet-based survey. The results show that for most panelists, the surgical indication for immediate laser is not obvious in more than 20% of stage 1 TTTS. However, following the answers of these panelists, if an immediate invasive procedure is warranted, it should always be laser, and never amnioreduction. The reasons for this are that: (1) laser is proven superior to amnioreduction in stages 1 and 2 combined; (2) a prior amnioreduction could compromise the outcome of a subsequent laser; (3) stage 1 TTTS carries a risk of progression of about 30–45%. Answers also show that if surgery is not clearly indicated, the best management strategy would be expectant weekly follow-up. However, a majority of specialists consider a short cervical length per se as an important argument for immediate laser, irrespective of the stage of the disease.
The main problem for analyzing previously published data is in the heterogeneity in the definition of TTTS itself, leading to conflicting points of view on the optimal treatment of stage 1 TTTS. According to the initial article by Quintero et al. (Reference Quintero, Morales, Allen, Bornick, Johnson and Kruger1999), TTTS is defined on ultrasound as polyhydramnios with a deepest vertical pool (DVP) >8 cm in the recipient twin, irrespective of gestational age at diagnosis, together with oligohydramnios with a DVP <2 cm in the donor twin. Concerned by the fact that this definition may not be appropriate for deciding on an invasive treatment, the Eurofetus trial opted for a more stringent definition of polyhydramnios, using an 8 cm cut-off before 20 weeks and 10 cm thereafter (Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004).
The Eurofetus trial aimed to compare percutaneous laser to amnioreduction and demonstrated, with proof beyond reasonable doubt, the overall superiority of laser over amnioreduction in severe TTTS using the previous definition, both in terms of twin survival and neurological morbidity. However, the data were insufficient for the post-hoc specific analysis of stage 1 disease. Indeed, 142 pregnancies were enrolled in this trial (72 in the laser arm and 70 in the amnioreduction arm), with only 11 pregnancies presenting with stage 1 (6 and 5 cases respectively), preventing any meaningful statistical sub-analyses.
This ongoing international randomized trial aims to compare two management strategies: the first relies on the overall conclusion from the Eurofetus trial and advocates immediate percutaneous fetoscopic surgery for all stages of TTTS, including stage 1 disease; the second is a conservative strategy, in which patients are monitored weekly until delivery or until progression warranting laser surgery. The primary end-point for this comparison encompasses both survival and neurological morbidity in a composite outcome, using a cluster-designed trial allowing the use of a per-fetus outcome rather than a per-pregnancy outcome as described later.
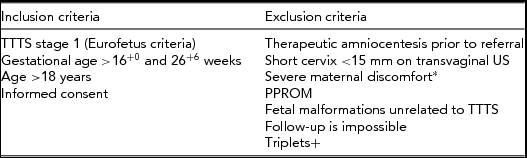
Eligible participants are women with monochorionic, diamniotic twin pregnancies presenting with stage 1 TTTS defined according to the Eurofetus criteria between 16+0 and 26+6 weeks of gestation. Patients with a cervix less than 15 mm on transvaginal scan (5th percentile) (Salomon et al., Reference Salomon, Nasr, Nizard, Bernard, Essaoui, Bussieres and Ville2008) or severe maternal discomfort are excluded as these may require immediate treatment. Similarly, patients presenting with PPROM or with fetal malformations will be excluded (Table 4).
TABLE 4 Inclusion and exclusion criteria of the stage 1 RCT
The purpose of this study is to compare immediate percutaneous laser surgery to conservative management in stage 1 TTTS (Figure 1). Surgery will be planned within 72 hours following randomization. Patients allocated to expectant management will be monitored weekly to ensure the absence of progression in disease or obstetrical worsening. The follow-up will be planned on randomization to ensure that patients allocated to conservative management will be monitored no more frequently than every 5–7 days. Progression to stage 2 and higher, or the occurrence of severe maternal discomfort as well as a cervical shortening <15 mm during follow-up, will warrant laser surgery between 16+0 and 26+6 weeks. In case of disease progression or maternal discomfort occurring after 26+6 weeks, treatment will comprise of amnioreduction and steroids or delivery. Other indications for steroids will be based upon local protocols. Otherwise, in non-progressive syndromes, follow-up will be sustained weekly up until 27+0 and every other week thereafter. A progression rate between 30–45% is expected from small retrospective studies of stage 1 TTTS managed expectantly (Dickinson & Evans, Reference Dickinson and Evans2004; O'Donoghue et al., Reference O'Donoghue, Cartwright, Galea and Fisk2007; Taylor et al., Reference Taylor, Denbow, Duncan, Overton and Fisk2000). Progression to stage 2 or above is defined according to the usual Quintero staging.
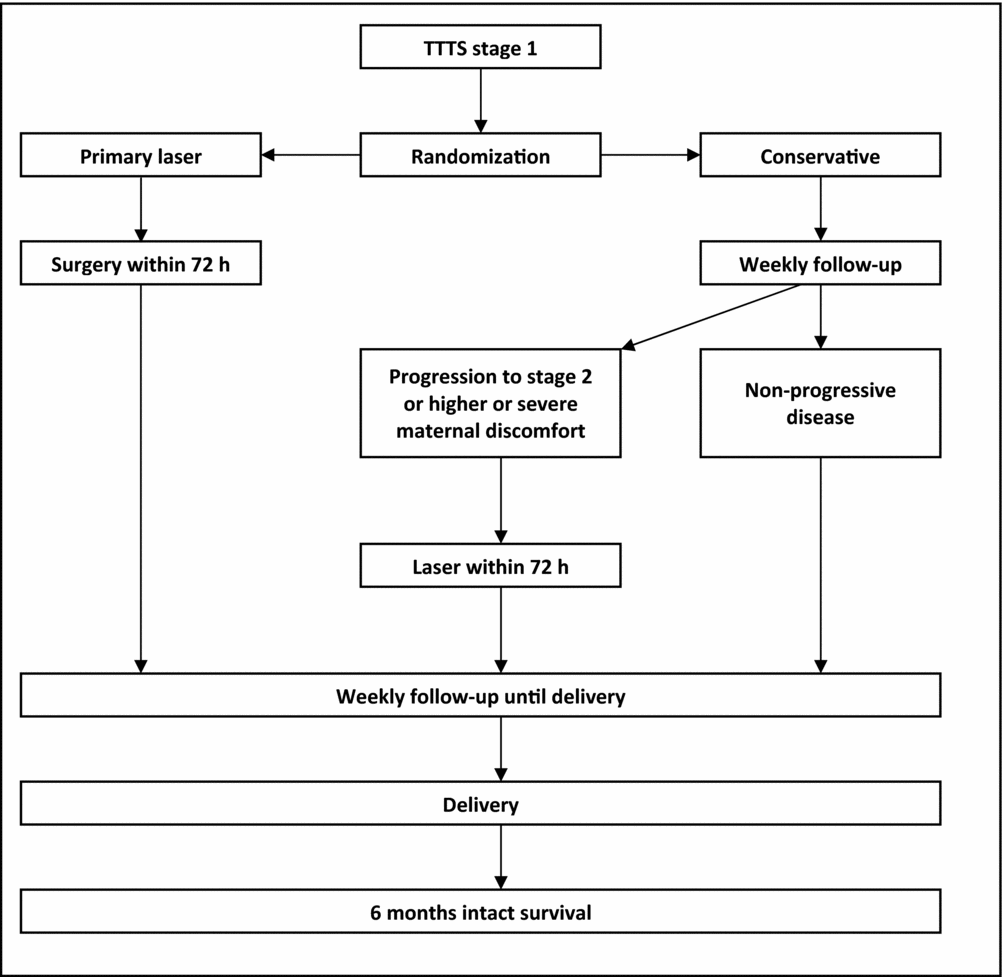
FIGURE 1 Design of TTTS1 randomized trial.
All babies born alive will be followed until the age of 6 months, corrected from estimated date of delivery. The primary end-point is overall intact survival at 6 months. This composite outcome characterizes the babies alive at 6 months without neurological sequelae. Neurological sequelae are defined as cystic periventricular leukomalacia, severe intraventricular hemorrhage (stage 3 or 4), blindness or deafness. Pragmatically, using this outcome in a trial consists of counting the number of babies alive and well at 6 months in each arm, taking into account the correlation between the twins of each pregnancy for a statistical comparison. This outcome is used for the calibration of the trial.
Secondary end-points comprise: 6 months and 2 years intact survival of both twins; perinatal, 6 months and 2 years survival of at least one twin and of both twins; complications of prematurity at 6 months and 2 years (necrotizing enterocolitis ≥stage 2, bronchopulmonary dysplasia, renal failure, retinopathy of prematurity, time spent in NICU); neurological morbidity at 2 years, as defined by any of: cerebral palsy according to the European CP network, blindness, severe deafness requiring amplification, or abnormal scores on the Bayley's test (a Bayley's test will be considered abnormal if the mental developmental indexes or psychomotor development indexes are under 70); maternal and obstetrical morbidity.
The trial has been powered according to a single bilateral test of two proportions difference in the rates of intact survival at 6 months between the two allocation groups. With α = 0.05, 1-β = 0.8, P1 = 60%, P2 = 75% meaning a clinically relevant difference of 15% between the groups, 200 fetuses or equivalently 100 pregnancies would be needed in each arm.









