Article contents
An extensive study on carbon nanomaterials electrode from electrophoretic deposition technique for energy storage device
Published online by Cambridge University Press: 28 March 2016
Abstract
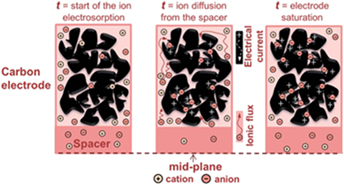
The development of energy storage device utilizing carbon nanomaterials possesses remarkably significant electrochemical performance. As compared to others, carbon nanomaterials including carbon black, graphene, activated carbon, and carbon nanotube have advantages in ion accessibility and specific surface area in which, more charged ions can access and transfer to the surfaces of material and thus have enhanced electrical charge storage performance. This manuscript briefly reviews the deposition of carbon nanomaterials from electrophoretic deposition technique which is good because of its simple, economical, versatility, and possibility of the thin film deposition on large substrate. The current state-of-the-art and performance of devices employing carbon as electrode material is also extensively discussed.
Keywords
- Type
- Invited Article
- Information
- Journal of Materials Research , Volume 31 , Issue 13: Focus Issue: Advances and Challenges in Carbon-based Tribomaterials , 14 July 2016 , pp. 1972 - 1982
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 6
- Cited by




