Article contents
Morphology and mesopores in photoelectrochemically active LaTiO2N single crystals
Published online by Cambridge University Press: 02 February 2016
Abstract
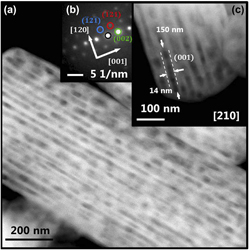
The mesoporous network within photocatalytically and photoelectrochemically active LaTiO2N (LTON) single crystals was investigated by electron microscopy techniques including electron diffraction and scanning transmission electron microscopy imaging. The perovskite-related oxynitride particles were obtained by thermal ammonolysis from monocrystalline micrometer-sized La2Ti2O7 (LTO) particles grown by flux-assisted solid state synthesis. Special attention was paid to the crystal transformation from the monoclinic layered LTO to the orthorhombic perovskite-related LTON within the monocrystalline particles. A detailed analysis of pore directions and pore sizes with respect to the LTON particle shape was performed. The pore formation mechanism taking place during thermal ammonolysis was discussed. Based on the mechanistic understanding of the transformation from the oxide to the oxynitride, a further extension of the mesoporous network toward higher surface areas was proposed for improved photoelectrochemical activity of oxynitride particles, while high crystallinity and particle sizes in the micrometer range continue to enable efficient charge transport.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 31 , Issue 11: Focus Issue: Advanced Materials and Structures for Solar Fuels , 14 June 2016 , pp. 1574 - 1579
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 11
- Cited by




