Diabetes prevalence is increasing in the United States,Reference Cheng, Imperatore, Geiss, Wang, Saydah and Cowie 1 and the appropriate management of patients with diabetes has become increasingly important for the prevention of hospital-acquired infections. Much has been published in recent years about the impact of diabetes on increased rates of surgical site infection (SSI) and the potentially related impact of hyperglycemia on SSI. Surgical site infections are estimated to have an annual financial impact of more than $3 billion dollars nationally and are the largest contributor to the overall cost of healthcare-associated infections.Reference Zimlichman, Henderson, Tamir, Franz, Song and Yamin 2 Efforts to reduce the rates of SSIs are becoming more urgent since the introduction of Centers for Medicare and Medicaid Services penalties for hospital readmission rates. An understanding of patient risk factors for SSI is key to these efforts because hospitals with a more vulnerable case mix are more likely to incur readmission penalties.Reference Joynt and Jha 3 Furthermore, the substantial prevalence of hospital-associated infections due to antibiotic resistant pathogensReference Sievert, Ricks, Edwards, Schneider, Patel and Srinivasan 4 highlights the importance of prevention in individuals at high risk of infection. To gain a greater understanding of the impact of pre-existing diabetes on the incidence of SSI, we performed a meta-analysis of risk factors for SSIs among patients undergoing surgery in US hospitals. We hypothesize that pre-existing diabetes is a significant contributor to the development of SSI, independently of hyperglycemia at the time of surgery. Secondarily, we hypothesize that hyperglycemia is itself an independent contributor to increased risk of SSI in surgical patients.
METHODS
A systematic literature search and meta-analysis was performed following MOOSE guidelinesReference Stroup, Berlin, Morton, Olkin, Williamson and Rennie 5 (Online Supplementary Material).
Data Sources and Searches
A systematic literature search was performed by 4 study investigators (M.S., C.K., H.N., R.E.) with questions referred to an adjudication team consisting of the study principal investigator (E.T.M.), 1 investigator with expertise in diabetes epidemiology (L.J.), and 1 investigator with expertise in infectious diseases and infection prevention (K.S.K.). The search was performed in PubMed and EMBASE using combinations of the search terms “risk factors,” “diabetes,” “glucose,” and “surgical site infections” from December 1985 to July 2015 (see Online Supplementary Material: Search Strategy). The starting date of the search, December 1985, was selected to correspond with the wide implementation of the Centers for Disease Control and Prevention (CDC) SSI surveillance guidelines. The search was inclusive of all study designs unless interventional control of glucose during the study prevented an assessment of the association between diabetes and SSI.
Study Selection
All abstracts were reviewed for eligibility, and the full-article texts of potentially relevant studies were reviewed in depth. Reference lists for all reviewed articles were hand-searched to identify additional eligible articles. Eligibility criteria for study inclusion were the following: (1) original US data; (2) adult participants; (3) utilized the CDC definition for SSIs; and (4) included measurable risk estimates of the association between diabetes and risk of SSI with 95% confidence intervals or provided adequate information to calculate risk estimates and their 95% confidence intervals. Review articles, meta-analyses, or non-English studies were excluded (Online Supplementary Material: List of Excluded Studies).
Eligible studies included adult patients undergoing surgical procedures of any type, using NHSN operative procedure categories to define surgical procedures. All comparative study designs (including observational, randomized controlled, retrospective, or prospective studies) were considered for inclusion provided they presented an assessment for the association between diabetes and SSI or the absolute patient numbers needed for the calculation of the measure. Each eligible study was required to include both diabetic and non-diabetic patients in the study population. Multiple publications on the same subject population were reviewed together and were restricted such that each patient population was included only once; this is notable particularly for multiple publications from large surveillance databases (eg, National Surgical Quality Improvement Program; see Online Supplementary Material: Excluded Studies). SSI was defined using criteria specified by the CDC for the purposes of surveillance and reporting.
Data Extraction
Measures for the association between pre-existing diabetes and SSI were collected from studies that ascertained the presence of diabetes prior to the time of surgery either through the patient’s medical record or hemoglobin A1c testing (HbA1c). Assessments of HbA1c levels were noted; however, not enough studies were identified to merit a separate meta-analysis based on this measure. Measures of the association between peri- or post-operative blood glucose levels were collected from studies that assessed thresholds of glucose levels. Studies that presented only comparisons of mean or median blood glucose levels were excluded from the analysis of peri- or post-operative hyperglycemia due to our inability to define the absolute number of patients with hyperglycemia in the infected and uninfected groups.
Data were abstracted onto standardized forms that included study characteristics, study population, type of SSI (superficial, deep incisional, or organ/space), crude and adjusted estimates, and confidence intervals. For each study, we recorded how diabetes was determined and whether blood glucose was measured prior to, during, or after surgery. Studies were assigned to the following categories based on the type of surgery: obstetrical and gynecological, colorectal, arthroplasty, breast, cardiac, spinal, or other. The abstraction team received training by the principal investigator, including the abstraction of multiple practice cases, before performing data abstraction. A subset of studies included was re-reviewed by 2 study investigators to ensure consistency.
Data Synthesis and Analysis
The most-adjusted estimate (ie, the adjusted odds ratio for the multivariate regression with the most variables) was used to generate summary estimates.Reference Petitti 6 Summary estimates and predictive intervals were calculated using a DerSimonian and Laird random-effects model for each estimate type. Confidence intervals were used for smaller analyses of diabetes and glucose combined models. The use of random-effects models was based on I2 values exceeding 30% in each overall fixed-effects analysis.Reference Higgins and Green 7 Funnel plots were generated to assess publication bias (data not shown) (Stata 11, StataCorp, College Station, TX). Sensitivity analysis was performed through the generation of stratified estimates and the use of multiple meta-regression analyses to assess the presence of meta-confounding by study characteristics including surgery type, study type, inclusion of body mass index (BMI) in the adjusted estimate, and diabetes prevalence in the study population. We determined a priori that the primary confounder of concern was BMI.
RESULTS
The combined search strategies identified 3,631 abstracts. All of these abstracts were reviewed, and the full texts of 522 articles were reviewed in depth; 3,109 studies were excluded during abstract review (Figure 1), and 428 studies were excluded during full-text review (Online Supplementary Material: List of Excluded Studies).

FIGURE 1 Flow diagram of search and selection processes.
Meta-analysis for Diabetes and SSI
A total of 90 studies provided estimates for the association between diabetes and SSI, including 2 studies that provided multiple estimates (Appendix Table 1). Included studies comprised a total of 866,427 procedures and 32,067 SSIs meeting CDC surveillance criteria. All studies were observational with the exception of 3 randomized controlled trials. We identified 14 studies (16%) that used prospectively collected data. Diabetes prevalence among included study populations ranged from 2% to 39% (median 17%). History of diabetes was ascertained by medical record review in all but 2 studies.Reference Latham, Lancaster, Covington, Pirolo and Thomas 8 , Reference Liang, Li, Avellaneda, Moffett, Hicks and Awad 9 No included studies differentiated between Type 1 and Type 2 diabetes.
The overall effect size for the association between diabetes and SSI was an odds ratio (OR) of 1.53 (95% predictive interval [PI], 1.11–2.12; I2, 57.2%) (Figure 2). Of the included studies, 38 (42%) provided estimates that were adjusted for potential confounding factors. When stratifying the meta-analysis by the availability of crude versus adjusted measures, the effect size was similar (OR, 1.68; 95% PI, 1.03–2.72; I2, 63.6%) for all available crude measures (71 studies); OR was 1.77 (95% PI, 1.13–2.78); I2, 71.1%) for all adjusted measures (38 studies). Funnel plots demonstrated greater evidence of potential publication bias for adjusted estimates (data not shown).

FIGURE 2 Meta-analysis of diabetes and surgical site infection, by surgery type.
Evaluation of Sources of Heterogeneity for Diabetes Estimate
Study design did not have a significant impact on the overall pooled estimate (P=.13 for retrospective vs prospective data collection; P=.41 for case-control vs cohort or interventional design). Prevalence of diabetes among study participants was also not a significant source of heterogeneity (P=.80). Among studies presenting estimates restricted to specific SSI classes, the combined OR was 1.95 (95% PI, 0.65–5.87) for deep SSIs (7 estimates from 6 studies) and the OR was 1.38 (95% PI, 0.66–2.88) for superficial SSIs (6 estimates from 5 studies).
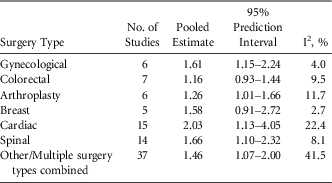
Among studies reporting on a single surgical category, the most common category was cardiac (15 studies) followed by spinal (14 studies) (Table 1). Estimates by surgery type for the association between diabetes and SSI ranged from 1.16 for colorectal surgeries (95% PI, 0.93–1.44) to 2.03 for cardiac surgeries (95% PI, 1.13–4.05) (Table 1). Meta-regression for impact of surgery type on the association between diabetes and SSI indicated that the combined SSI effect was higher for cardiac surgery than for all other surgery categories (P=.001). BMI was hypothesized a priori to be an important confounder in the association between diabetes and SSI. Study estimates were stratified according to whether the presented measure controlled for the effect of BMI. The estimate pooled from the 20 studies that controlled for BMI was higher than that pooled from those that did not; however, this factor was not significant when evaluated by meta-regression (P=.79).
TABLE 1 Pooled Estimates of the Association between Diabetes and Surgical Site Infection by Surgery Type
Meta-analysis for Blood Glucose and SSI
In total, 16 studies were available to assess the association between hyperglycemia and SSI, with 10 papers (n=27,844 procedures) including pre- or intraoperative assessments of blood glucose levels and 11 papers (n=32,625 procedures) including postoperative assessments of blood glucose levels. We observed a wide range in the threshold for defining hyperglycemia. Of 10 studies assessing pre-operative blood glucose, 6 used a threshold of 200 mg/dL, and the remaining 3 studies used thresholds of 125 mg/dL (2 studies), 180 mg/dL (1 study), and 100 mg/dL (1 study). Of the 11 studies assessing post-operative blood glucose, 5 used thresholds of 200 mg/dL, with the remaining studies using lower thresholds ranging from 125 mg/dL to 180 mg/dL. One paper presented a composite exposure of pre- or postoperative hyperglycemia, and this estimate was included in both pooled calculations.Reference Olsen, Nepple, Riew, Lenke, Bridwell and Mayfield 10 The overall estimate for the association between elevated blood glucose and SSI in the pre- or intraoperative period was OR=1.88 (95% Predictive Interval [PI], 0.66–5.34) (Figure 3). The overall estimate for the association between elevated blood glucose in the post-operative period and SSI was 1.45 (95% PI, 0.77–3.04) (Figure 4).

FIGURE 3 Meta-analysis of pre-operative hyperglycemia and surgical site infection.

FIGURE 4 Meta-analysis of post-operative hyperglycemia and surgical site infection.
Only 3 studies presented multivariate models adjusting for blood glucose levels and diabetes in the same model.Reference Latham, Lancaster, Covington, Pirolo and Thomas 8 , Reference Olsen, Nepple, Riew, Lenke, Bridwell and Mayfield 10 , Reference Wilson and Sexton 11 These studies used thresholds of ≥200 mg/dLReference Latham, Lancaster, Covington, Pirolo and Thomas 8 and ≥126 mg/dLReference Wilson and Sexton 11 to define elevated preoperative glucose levels or combined pre- and postoperative thresholds into a single definition.Reference Olsen, Nepple, Riew, Lenke, Bridwell and Mayfield 10 Pooled estimates of the association between diabetes and SSI, controlling for glucose level was OR=2.55 (95% confidence interval [CI], 1.70–3.82). Pooled estimates of the association between elevated glucose level and SSI, controlling for a history of diabetes was OR=2.22 (95% CI, 1.36–3.60).
DISCUSSION
Consistency of Included Studies
We limited our analysis to studies performed at U.S. hospitals after 1985 in an effort to narrow our review to surgeries evaluated with the standard SSI surveillance methods and definitions required by the CDC. We observed some variation in the definitions for hyperglycemia among included studies. Approximately half of the studies used thresholds that met or were more conservative than those proposed by the Society of Thoracic SurgeonsReference Lazar, McDonnell, Chipkin, Furnary, Engelman and Sadhu 12 and the American Diabetes Association. 13 The remaining studies used a slightly higher threshold of 200 mg/dL to define hyperglycemia, and it is possible that this variation may have introduced heterogeneity into our combined estimates for hyperglycemia.
Almost all included studies used medical record review to assess a patient’s reported history of diabetes. This data may be prone to error and may not reliably identify all patients with diabetes or assess the degree to which an individual patient’s diabetic condition is adequately controlled. Likewise, the assessment of diabetes for use with the revised surgical risk index from the Centers of Disease Control does not recommend the use of HbA1c or other markers of severity of diabetes to gather risk information.Reference Mu, Edwards, Horan, Berrios-Torres and Fridkin 14
Generalizability of Study Estimate
Our requirement that all studies be based in the United States excluded available data from other countries; however, it allowed us to strictly assess SSIs using CDC definitions from hospitals participating in standardized surveillance procedures. While specific quality ratings were not performed for each study, we explored several potential sources of heterogeneity in our pooled estimates through the use of meta-regression. Our stratum-specific estimates show a very consistent association between diabetes and SSIs across categories, including study characteristics, and after controlling for BMI. We were unable to assess variation due to SSI surveillance practices in different hospitals. While studies using active follow-up protocols would be expected to have increased SSI rates, we do not expect that this effect would be differential by diabetes status. Our pooled estimates are based on the use of the most adjusted estimate available in each study.Reference Petitti 6 To assess the impact of this rule, summary estimates were generated separately for all available crude effects and all available adjusted effects, and the findings in these models were similar to the most-adjusted models. The funnel plot for the adjusted estimates indicates the possible presence of publication bias for these estimates in this body of literature (data not shown). This bias is likely due to the tendency of authors to publish only those variables that are significant in multivariate analyses. Given our findings of an association between diabetes and SSI in almost every category of surgery type, it is likely that non-significant findings for diabetes in smaller studies may be due to insufficient sample size in individual studies, rather than a lack of underlying impact. For that reason, it may be prudent to include diabetes as an a priori hypothesized risk factor in future studies with the inclusion of diabetes in adjusted models for risk of SSIs.
Interpretation of Findings
Our finding of increased SSI among patients with diabetes was consistent across surgery types, with the exception of obstetrical and gynecological surgery which was based on 2 studies conducted at the same hospital. The surgery-specific findings were statistically significant for arthroplasty, breast, cardiac, and spinal surgeries; the actual pooled estimate was highest among patients undergoing cardiac surgery. These findings contrast with the analysis of National Healthcare Safety Network data that served as the basis for the revised procedure-specific SSI risk-adjustment calculations. This analysis found diabetes to only be associated with SSI for spinal fusion or refusion.Reference Mu, Edwards, Horan, Berrios-Torres and Fridkin 14 In patients with diabetes receiving colorectal resection, glucose levels were consistently higher in patients with an SSI compared with uninfected patients, even when mean glucose levels were below 200 mg/dL in those with or without SSI.Reference Sehgal, Berg, Figueroa, Poritz, McKenna and Stewart 15 Similar findings have been reported in patients undergoing laminectomy.Reference Friedman, Sexton, Connelly and Kaye 16 Elevated blood glucose has been found to be associated with increased rates of infection in orthopaedic spine surgery,Reference Caputo, Dobbertien, Ferranti, Brown, Michael and Richardson 17 cardiac surgery,Reference Wilson and Sexton 11 , Reference Furnary, Zerr, Grunkemeier and Starr 18 , Reference Zerr, Furnary, Grunkemeier, Bookin, Kanhere and Starr 19 and colorectal and bariatric surgeryReference Kwon, Thompson, Dellinger, Yanez, Farrohki and Flum 20 ; however, this association has not been consistently found.Reference Hardy, Nowacki, Bertin and Weil 21 , Reference Jeon, Furuya, Berman and Larson 22
The notion that diabetes is a significant contributor to SSI risk through mechanisms other than hyperglycemia at the time of surgery is supported by the few studies that included both glucose levels and history of diabetes in the multivariate models. In the pooled estimate from these studies, the magnitude of the effect of diabetes was stronger than that of our primary analysis that included all eligible studies. The results of an interventional study by Trussel et alReference Trussell, Gerkin, Coates, Brandenberger, Tibi and Keuth 23 corroborates this finding. Diabetes remained a significant risk factor for SSI with an odds ratio of 4.71 despite the implementation of a patient care pathway targeting glucose control during the time of surgery and resulting in successfully reducing the overall rates of infection. However, we identified few studies suitable for analysis of potential independent effects of diabetes and hyperglycemia on SSI.
CONCLUSIONS
In our study, we found a significant association between diabetes and SSI that was consistent across multiple types of surgeries and after controlling for BMI. While we also confirmed an association between both pre- and post-operative hyperglycemia and SSI, history of diabetes remained a significant risk factor in meta-analyses of studies that controlled for hyperglycemia.
Furthermore, we found diabetes to be a significant contributor to the risk of SSIs, potentially beyond its role in causing hyperglycemia during or after surgery. The reasons for this finding are unclear. It is possible that diabetes is a marker for other conditions that may put a patient at risk of infection, including vascular changes and white blood cell dysfunction. In addition, the occurrence of perioperative hyperglycemia and subsequent immune suppression is affected by the complex contributions of factors in addition to the diabetic history of the patient, including physiologic stressors and exogenous glucose administration.Reference Russo 24 Although we were able to assess the confounding effect of body mass index and found no effect on our conclusions, our ability to fully assess potential confounders in the current meta-analysis is limited by the variables assessed in the original studies. However, the most adjusted estimate from each study was used in the final analysis, which should benefit from control of other confounding variables at the individual study level. Our findings point to several directions for future research. Few studies have assessed whether a more detailed assessment of diabetes severity would improve the management of SSI risk in these patients. Secondly, few studies address the cause of the infection, and thus we are unable to rule out whether the increased risk of SSI among diabetics is related to differences in bacterial etiology.
Overall, these results support the consideration of diabetes as an independent risk factor for SSIs for multiple procedure types and continued efforts are needed to improve surgical outcomes for diabetic patients.
ACKNOWLEDGMENTS
Financial support. This work was supported by the National Institutes of Health (grant no. K01AI099006 to E.T.M.).
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/ice.2015.249
APPENDIX TABLE 1 Articles Included in 3 Meta-analyses of Diabetes and Pre- and Postoperative Hyperglycemia











