Introduction
Population monitoring plays an important role in conservation biology. Long-term monitoring is valuable for detecting population declines at an early stage, enabling research and management efforts to be allocated towards the most vulnerable species and habitats especially when funds are limited. Additional data on environmental variables enables potential causes of change to be evaluated. Furthermore, ongoing monitoring facilitates continual assessment of management effectiveness (Thomas Reference Thomas1996, Thomas and Martin Reference Thomas and Martin1996).
Aldabra Atoll, a UNESCO World Heritage Site, Important Bird Area (IBA; Rocamora and Skerrett Reference Rocamora, Skerrett, Fishpool and Evans2001), Ramsar Wetland Site of International Importance and Marine Protected Area, is part of the Seychelles archipelago in the south-western Indian Ocean. Aldabra has been strictly protected since 1976, was inscribed on the UNESCO World Heritage list in 1982 and has been managed for research and conservation since 1979 by the Seychelles Islands Foundation (SIF). A key component of SIF's programme of research on Aldabra is regular long-term monitoring of various taxa and ecosystems which can help to identify trends or changes, pinpoint causes of disturbance and guide action to reverse negative changes (Niemi et al. Reference Niemi, Hanowski, Lima, Nicholls and Weiland1997, Gregory et al. Reference Gregory, Gibbons, Donald, Sutherland, Newton and Green2004).
Aldabra’s main islands host several habitat types and vertebrate communities. Notably, unlike all tropical islands of comparable size in the Indian Ocean, Aldabra had until very recently no introduced avifauna. An introduced population of a few hundred Madagascar Fodies Foudia madagascariensis, discovered in March 2012 and restricted to a small part of the atoll is in the process of being eradicated (SIF unpubl. data); and a single introduced Red-whiskered Bulbul Pycnonotus jocosus was culled in July 2013 with no further sightings of this species (Bunbury et al. Reference Bunbury, Brandis, Currie, Jean-Baptiste, Accouche, Souyave, Haupt and Fleischer-Dogley2013a). The recent invasions by both species are likely to originate from the neighbouring island of Assumption where they were introduced in the 1970s. To preclude the possibility of reinvasion to Aldabra, an eradication programme led by SIF was started on Assumption in early 2012 and is currently in an advanced stage for both species. All other landbirds are considered endemic or native to Aldabra, with at least two endemic species (Aldabra Drongo Dicrurus aldabranus, Aldabra Fody Foudia aldabrana) and nine sub-species that originate from the Madagascar / Comores region. The Aldabra Fody was formerly considered a subspecies of F. eminentissima endemic to Aldabra; however, based on genetic evidence it has recently been elevated to species status (Safford and Hawkins Reference Safford and Hawkins2013; also see Warren et al. Reference Warren, Bermingham, Bourgeois, Estep, Prys-Jones, Strasberg and Thébaud2012). Hence its current conservation status as ‘Least Concern’ (IUCN 2014) requires reassessment to acknowledge the need to preserve the unique genetic diversity of this isolated population.
Landbirds occur in all terrestrial habitats of Aldabra and most are widely dispersed around the atoll. The landbird monitoring programme was set up to monitor the abundance and spatio-temporal dynamics of Aldabra’s landbirds. Here, we use the monitoring data to investigate long-term trends of abundance of seven species: the Comoro Blue pigeon Alectroenas sganzini minor, Madagascar Turtle-dove Streptopelia picturata coppingeri, Madagascar Bulbul Hypsipetes madagascariensis rostratus, Aldabra Drongo, Aldabra Fody, Souimanga Sunbird Cinnyris souimanga aldabrensis and Madagascar White-eye Zosterops maderaspatanus aldabrensis. Our specific aims are to: (1) determine the association between location, season and habitat type with the abundance of these landbird species; (2) detect whether there are abundance trends or changes of conservation concern; (3) assess the influence of monitoring methods on results; and (4) review the strengths and weaknesses of the current monitoring methods. Ultimately, we aim to use the above to improve the reliability of results and the conservation effectiveness of the Aldabra landbird monitoring programme.
Materials and Methods
Study area
Aldabra Atoll (9°24'S, 46°20'E; Figure 1) is a large (34 × 14.5 km; total land area: c.152.6 km2; SIF, unpubl. data) raised coral atoll consisting of a rim of four main islands (Picard, Polymnie, Malabar and Grande Terre), and 42 named and many unnamed smaller lagoon islands (Stoddart et al. Reference Stoddart, Taylor, Fosberg and Farrow1971), forming part of the Seychelles in the south-western Indian Ocean. Aldabra has a mosaic of habitats including coastal scrub, mixed scrub, dense patches of Pemphis acidula, open savannah-like areas of grassy turf, beach and dune systems, limestone rock and mangroves (Stoddart and Fosberg Reference Stoddart, Fosberg and Stoddart1984). The atoll's tropical, semi-arid climate is determined by changes in wind direction which in turn influence rainfall: May to October is the dry season with strong south-east trade winds blowing, while November to April is the wetter north-west season with lighter winds (Farrow Reference Farrow1971, Prys-Jones and Diamond Reference Prys-Jones, Diamond and Stoddart1984). More than 75% of the annual rain falls between December and April and this is the main breeding period for Aldabra's landbirds (Prys-Jones and Diamond Reference Prys-Jones, Diamond and Stoddart1984; SIF unpubl. data). Insect abundance is highly correlated with rainfall, peaking within the first month of the rains (Frith Reference Frith1975). Many of Aldabra's landbirds (turtle-doves, bulbuls, fodies and white-eyes) are generalists, with diets comprising fruits, seeds and insects. Specialists include the blue pigeon (frugivorous), drongo (insectivorous and carnivorous) and the sunbird (nectar and insects) (Prys-Jones and Diamond Reference Prys-Jones, Diamond and Stoddart1984).
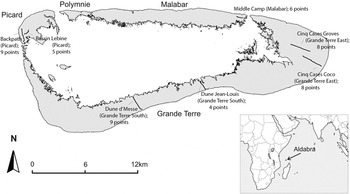
Figure 1. Location of Aldabra Atoll in the Indian Ocean (inset) with the sites of the seven landbird monitoring transects (Picard Back Path, Picard Bassin Lebine, Middle Camp, Cinq Cases Groves, Cinq Cases Coco, at the four different locations (Picard, Malabar, Grande Terre South and Grande Terre East). The number of points for each transect are given.
Monitoring methods
At seven locations on Aldabra, count points (200 m apart) for landbird monitoring were set out on existing trails, hereafter referred to as transects. These transects of varying lengths (from 800 m [4 points] to 1,800 m [9 points]; Figure 1) were surveyed monthly from 2002 to 2013 on the islands of Grande Terre, Malabar and Picard (Figure 1). To analyse densities per area, Grande Terre (GT) was split into 'GT East' and 'GT South', creating four 'locations': Picard (two transects), Malabar (one transect), GT East (two transects) and GT South (two transects). Transect monitoring starts at 07h00 (or as soon as there is enough light to distinguish birds) and finishes before 09h00. Counts were done by at least two observers standing back-to-back for 360° coverage, who communicated to avoid double counting. The identity and number of all landbirds seen or heard within a 25-m radius (estimated visually) of the point for four minutes (with no settling down period) was recorded. Flying birds are counted only if they fly through the 25-m radius area. Transects were walked quietly to limit disturbance, and communication between observers was quiet. Occasionally, due to resource limitations (staff, transport), not all aspects of the protocol could be adhered to; e.g. only one observer, monitoring starting later than 07h00.
Vegetation scores
Of Aldabra's 206 plant species, 80% are indigenous and 20% are endemic (Renvoize Reference Renvoize1971). Vegetation assessments of each transect were done in June and August 2012: each point was assigned one of four main vegetation types: (1) Standard mixed scrub: continuous scrub cover with few gaps, dominated by Acalypha claoxyloides Polysphaeria multiflora, Tarenna supra-axillaris, Euphorbia pyrifolia, Ochna ciliata and Mystroxylon aethiopicum with little or no ground layer vegetation; (2) Open mixed scrub: savannah-like mixed scrub dominated by Polysphaeria multiflora, Ochna ciliata, Mystroxylon aethiopicum, Maytenus senegalensis and Apodytes dimidiata, but with a wide range of other woody species present such as Erythroxylum acranthum, Canthium bibracteum, Flacourtia ramontchi and Terminalia boivinii. The cover is non-continuous in a landscape dominated by rocks, turf or grass; (3) Pemphis scrub: dense vegetation dominated by the coastal shrub Pemphis acidula, and (4) Coastal scrub: open coastal mixed scrub, dominated by Scaevola taccada or other wooded vegetation (Gibson and Phillipson Reference Gibson and Phillipson1983, SIF unpubl. data).
Statistics
The dataset included 1,029 events, in each of which 13 bird species were counted (n = 13,377 records). A Poisson distribution was used to fit the data. Due to many zero counts in which no birds of a particular species were counted, the data were positively skewed (skewness value: 3.27 ± 0.02). To avoid zero inflation of the models (Zuur et al. Reference Zuur, Ieno and Elphick2010) we included only species for which the dataset contained more positive counts than zero counts (blue pigeon, turtle-dove, bulbul, drongo, fody, sunbird and white-eye; n = 7,203 records). The Aldabra landbirds for which zero counts dominated were not included in this analysis (Aldabra White-throated Rail Dryolimnas cuvieri aldabranus, Madagascar Coucal Centropus toulou insularis, Madagascar Sacred Ibis Threskiornis aethiopicus bernieri, Madagascar Kestrel Falco newtoni aldabranus, Madagascar Nightjar Caprimulgus madagascariensis aldabrensis and Pied Crow Corvus albus) and require different monitoring methods.
We used hierarchical multi-level mixed models (MLWin; Rashbash et al. Reference Rashbash, Steele, Browne and Prosser2008) with 'transect' (level 2) and 'monitoring event' (level 1) nested as random effects to avoid pseudoreplication. To explore trends in bird counts, we constructed a model with all species pooled, as well as separate models for all species, with mean count per transect as dependent variable, and fitted the independent variables 'year' (continuous), 'season' (wet = 0, dry = 1), 'location' (Picard = 0, Grande Terre East (GTE) = 1, Grande Terre South (GTS) = 2, Malabar = 3), 'end time' (continuous), 'monitoring duration' (continuous), 'number of observers' (0 = one observer, 1 = two observers, 2 = ≥ 3 observers) as main effects, and an interaction between 'season' and 'location', with 0 as reference category.
Information on weather was not available for all events, therefore we constructed a separate model in a sub-set of data of the seven species from which climatic data – collected by the observers just before starting the transect – was available (237 events (atoll-wide), n = 1,659 records, from November 2010 onwards) with 'weather' (sunny = 0, overcast = 1) and 'wind' (calm = 0, moderate = 1, strong = 2), fitted as fixed factors in addition to the other above-mentioned variables.
To examine the influence of habitat type on the counts, we used a dataset that included count results per point rather than means per transect (n = 44,109 records). This enabled us to investigate vegetation composition for each point, as vegetation type varied along transects. We tested the influence of vegetation type (with 'standard mixed scrub' as reference category) for all bird species pooled, as well as for each species separately, in models fitted with the same variables and interactions as in the models above. Percentages of change in population trends were calculated and power analyses using SPSS v.21 (IBM) were conducted for each species to identify with how much confidence changes in population indices can be detected over the study period with the available sample size.
Results
Trends in abundance
There was an overall increase in landbird abundance from 2002 to 2013 (ßyear = 0.05 ± 0.004, P < 0.001; Table 1, Figure 2a, Table S1 in the online supplementary material), with only the drongo showing no increase (Figure 2d). Results of the power analyses exceeded the recommended acceptable power level of 0.8 (Hair et al. Reference Hair, Tatham, Anderson and Black2006) for all species except the blue pigeon, drongo and fody (Figure 2). Only turtle-doves showed spatial differences in abundance over time: the rate of increase was steeper on Grande Terre (both locations) than on Malabar and Picard (ßyear.GTE = 0.05 ± 0.02, ßyear.Malabar = -0.002 ± 0.02, ßyear.GTS = 0.09 ± 0.03; χ2 3 = 16.36, P < 0.001). No location (independent of interactions) had consistently higher numbers of landbird species (Table 1). Malabar had higher numbers of blue pigeons, turtle-doves and drongos than Grande Terre (both locations), but fewer sunbirds and white-eyes. White-eyes were most abundant on Grande Terre (both locations), and sunbirds were most abundant at Grande Terre South. Fodies were most abundant at Grande Terre East and least abundant on Grande Terre South and Malabar.
Table 1. Final models showing associations of spatial and temporal variables with abundance of seven focal landbird species on Aldabra, derived from a hierarchical linear mixed-modelling procedure with Poisson distribution and stepwise backward elimination of non-significant terms. Slopes (ß; fixed variables) and variances (σ; random variable 'transect') are given with their standard errors. GTS = Grande Terre South, GTE = Grande Terre East. An extended version of this Table, including slope estimates for all categories, can be found in the Online Supplementary Materials.


Figure 2. Patterns of landbird count results over the period 2002–2013, for seven focal landbird species on Aldabra, shown as mean ± SE. number of birds counted per transect and their regression line with 95% confidence intervals. Equations in each panel represent the final model predictions of Table 1. Percentages of change and the results of a power analysis are provided, along with the equation of the slope.
Drongos, fodies and white-eyes were more abundant in the wet than the dry season, while turtle-doves were more abundant in the dry season. The remaining three species showed differing patterns per location (Table 1; Figure 3).

Figure 3. Landbird counts at the different locations during the wet (breeding) season and the dry (non-breeding) season of seven focal landbird species on Aldabra, presented as residuals ± SE of the final models (Table 1).
Associations of location, season and habitat type with abundance
There was no overall difference in landbird abundance between locations (χ2 3 = 6.53, P = 0.09), but post-hoc tests showed that abundance at Grande Terre South was lower than at Grande Terre East (P = 0.026) and Malabar (P = 0.041). Overall, more birds were counted in the wet than the dry season (ßwet season = –0.15 ± 0.04, P < 0.001). Post-hoc analyses showed this pattern on Picard and Grande Terre, but on Malabar the opposite pattern occurred, with abundance in the dry season being higher than in the wet season (χ2 2 = 45.2, P < 0.001). Long-term patterns generally did not differ between locations (χ2 3 = 6.71, P = 0.08).
In general, most birds were counted in open mixed scrub, followed by standard mixed scrub, Pemphis scrub and coastal scrub (χ2 3 = 850.3, P < 0.001; Figure 4a). Counts were highest for most species in mixed scrub (Figure 4), but for sunbirds and white-eyes relatively high counts were recorded in Pemphis scrub (Figure 4f, h). Counts in coastal scrub were generally low.

Figure 4. Mean landbird count results per count point in different vegetation types, shown for all birds combined and for all seven focal landbird species separately, presented as residuals ± SE of the final models shown in Table 1. Groups with the same letter do not differ significantly (P > 0.05).
Monitoring methods
The time of monitoring affected the results, with fewer birds counted in later monitoring sessions (ßend time = –0.14 ± 0.03, P < 0.001; accounting for length of the monitoring session).
More birds were counted with more observers in the session (compared to reference category of one observer: ß2obs.= 0.13 ± 0.04, ß≥3obs.= 0.22 ± 0.05, χ2 2 = 21.7, P < 0.001; all post-hoc tests χ2 1 > 9.06, P < 0.001). Blue pigeons, turtle-doves and white-eyes were most affected by monitoring circumstances: count results for these species were negatively related to end time of counts and positively related to the number of observers (Table 1).
Count results were associated with weather conditions, with fewer birds counted on cloudy compared to sunny days (ßcloudy = –0.12 ± 0.05, P = 0.005) and during strong winds compared to calm conditions (ßmoderate = < 0.001 ± 0.04, ßstrong = –0.27 ± 0.09, χ2 2 = 5.38, P = 0.02, all post-hoc tests for category 'strong' χ2 1 > 9.48, P < 0.002).
Discussion
Aldabra’s landbird monitoring data can be used as an index of the relative changes in the individual bird populations, but not as a measure of population sizes per se (Gregory et al. Reference Gregory, Gibbons, Donald, Sutherland, Newton and Green2004) or to compare absolute densities, as the method used does not account for variation in detectability between species (Dawson and Bull Reference Dawson and Bull1975, Bibby et al. Reference Bibby, Burgess and Hill1992, Wheater et al. Reference Wheater, Bell and Cook2011).
One notable result is the rise in abundance of all species except the drongo (Table 1, Figure 2). It is unlikely that this increase was caused by gradual changes in monitoring, as we accounted for monitoring circumstances and there was substantial variation among years. The long-term increases suggest that, at least in the past decade, Aldabra's landbird populations were either not at carrying capacity, or that they are recovering following declines that could be related to abiotic (e.g. climatic) and biotic (e.g. vegetation) factors, or to former human exploitation for species such as the turtle-dove (Benson and Penny Reference Benson and Penny1971). The stable drongo population may indicate that the population is at carrying capacity, which may be related to the birds' relatively large territory sizes (2.25–4.5 ha; Collar and Stuart Reference Collar and Stuart1985, Rocamora Reference Rocamora2013). Alternatively, low reproductive success may be preventing population increase: Aldabra Drongo breeding success has been consistently recorded as low (20–25%; Frith Reference Frith1979a, François and Fanchette Reference François and Fanchette2003, Šúr Reference Šúr2012, SIF unpubl. data 2014), due primarily to predation by black rats Rattus rattus and possibly other birds (François and Fanchette Reference François and Fanchette2003). Aldabra’s other landbirds, however, experience similarly high nest predation (Frith Reference Frith1979b, Šúr Reference Šúr2012) so this seems unlikely to be the sole cause. Landbird nest monitoring on Aldabra is ongoing to determine reproductive success of different species and the main causes of nest failure.
The relative population increases of the other landbirds from 2002 to 2013, most marked in sunbirds and white-eyes (c.100% increase), blue pigeons (c.95% increase), turtle-doves (c.90% increase) and fodies (c.83% increase), could be due to vegetation changes over time. One major change over (and before) this period which has affected the vegetation of Grande Terre is the eradication of feral goats. Goats were introduced to Aldabra over 150 years ago and occurred on all four major islands but by 1995 they remained only on Grande Terre (Burke Reference Burke1988, Coblentz et al. Reference Coblentz, Main and van Vuren1990). Approximately 250 goats were culled on Grande Terre between 2000 and 2005 and, by 2007, their distribution was restricted to eastern Grande Terre (Von Brandis Reference Von Brandis2007). Over 200 goats were culled in 2008, 17 in 2009, and the last few in 2012 when Grande Terre was declared goat-free (Bunbury et al. Reference Bunbury, Mahoune, Raguain, Richards and Fleischer-Dogley2013b). Goats have detrimental impacts on vegetation structure, composition and regeneration (Coblentz et al. Reference Coblentz, Main and van Vuren1990, Rainbolt and Coblentz Reference Rainbolt and Coblentz1999). The long presence of goats on Aldabra, followed by their gradual reduction and ultimate elimination from the ecosystem could plausibly explain the suppression and subsequent recovery of some landbird populations. Our results support this, with the increase of turtle-doves and bulbuls being more marked on Grande Terre than on Malabar or Picard.
Direct predator pressure is also expected to influence bird abundance. The black rat is the main introduced nest predator present on all of Aldabra’s islands (Stoddart Reference Stoddart1971, Prys-Jones Reference Prys-Jones1979, Prys-Jones and Diamond Reference Prys-Jones, Diamond and Stoddart1984). Feral cats, introduced by early settlers (Stoddart Reference Stoddart1971), also prey on adult birds but occur only on Grande Terre. Blue pigeons, turtle-doves and drongos were less abundant on Grande Terre than on the cat-free islands. Repeated observations of thousands of turtle-doves and hundreds of blue pigeons flying from Grande Terre to Malabar in the late afternoons suggest that these species forage on Grande Terre during the day and roost on Malabar (Prys-Jones and Diamond Reference Prys-Jones, Diamond and Stoddart1984, SIF unpubl. data). In the early mornings, when the counts are conducted, it is possible that the doves are still migrating towards Grande Terre, which would result in lower counts on this island. A large proportion of the blue pigeon and turtle-dove counts derive from flying groups (SIF staff pers. obs), which makes it worth considering excluding flying birds from counts as these movements may not reflect local distribution patterns. Fruit availability may play a role in these short-distance migrations; blue pigeons and turtle-doves are obligate frugivores, and fruits of the aseasonal Ficus spp. that is abundant on Grande Terre are an important food source for both species when other fruits are scarce (Prys-Jones and Diamond Reference Prys-Jones, Diamond and Stoddart1984).
In addition to the overall increasing long-term trends, there are also interesting differences between species in the steadiness of these trends; with some species showing more inter-annual fluctuations than others. For example, sunbirds show large increases followed by smaller decreases, and particularly in 2006 many species showed a steep change in numbers. The meteorological data from Aldabra indicates that 2006 was a very wet year, which was probably linked to the Indian Ocean Dipole (IOD) positive event (Cai et al. Reference Cai, Pan, Roemmich, Cowan and Guo2009). In the harsh and generally arid environment of Aldabra where food is often limited, the effects of climatic changes (rainfall) may be magnified resulting in rapid responses in landbird population dynamics.
Another interesting result is that the interaction between season and location (which could be generally due to spatial variation in rainfall; Frith Reference Frith1979a) differed between species. This difference might be linked to detectability, which varies not only between species but between seasons. Only fodies and white-eyes showed higher counts in the wet (breeding) season than in the dry season. White-eyes in particular were primarily detected by sound, and seasonal changes in leaf coverage are less influential. Territoriality might influence fody counts, since males are often highly visible even in dense vegetation, perching and displaying prominently. Turtle-doves showed the opposite pattern, with higher counts in the dry than the wet season. Lower foliage coverage in the dry season may partially account for this result but it may also be influenced by delayed dove migration times, due to reduced and later daylight hours in the dry season, whereas the timing of monitoring remains the same throughout the year.
Most birds were counted in mixed scrub; these habitats have the highest floral species and insect richness (Frith Reference Frith1975, Reference Frith1979a), and therefore the most varied phenology and highest year-round availability of food. Pemphis scrub was more frequented by sunbirds and white-eyes than by other species.
Review of monitoring methods
Our analysis enables a critical review of the Aldabra landbird monitoring programme by identifying its strengths and weaknesses. Strengths include its ease of application, minimal training needs (partly because Aldabra has few landbird species) and lack of constraints from observer bias, which is arbitrary due to the large dataset, high monitoring frequency and large number of observers randomly combined in teams of two over the monitoring events.
Variability in detectability (between species, season) is a disadvantage of the current monitoring programme. For example, very vocal birds (sunbirds, white-eyes, bulbuls) are more likely to be counted than less vocal birds (turtle-doves, drongos) and some species show substantial seasonal variation in vocalisations. Recording whether birds are seen and/or heard, or introducing an index of detectability (Bibby et al. Reference Bibby, Burgess and Hill1992) to detect birds not only within, but also beyond a 25m radius would allow comparison of abundance between different species and help to interpret temporal changes in observation frequency. Applying successively longer radii around each point and using Distance sampling (Buckland et al. Reference Buckland, Anderson, Burnham and Laake1993) could be of use to calibrate the single-distance point counts used in the current monitoring programme. Our results indicate that the monitoring programme would benefit from excluding flying turtle-doves and blue pigeons to ensure that local distribution patterns are monitored rather than flight paths. The repeatability of the results is also higher with a consistent team of two observers (as one observer cannot cover a 360° observation circle, and more than two observers might increase the risk of double counting) and if the counts are done in calm dry conditions and finished before 09h00.
In applying our results to management, it is important to consider that sudden changes in population indices are currently difficult to identify due to considerable annual variation in the data. It therefore requires several years of data to confirm a declining trend requiring management intervention. The results from power analyses can help in judging to what degree of confidence an annual increase, gathered from a certain sample size, can be interpreted as a part of a long-term trend. Particularly for the four species that showed a power value of > 0.8, it is likely that the increases measured are genuine and sample sizes are appropriate (Hair et al. Reference Hair, Tatham, Anderson and Black2006). For example, the reliability of detecting genuine population increases is higher for the Madagascar Bulbul (for which a 41% increase was measured over 11 years with a power of 0.98 ± 0.01) than for the blue pigeon (for which an 86% increase was measured over 11 years with a power of 0.68 ± 0.09). Furthermore, the efficiency of this monitoring programme could be improved by decreasing the monitoring frequency, potentially increasing the number of transects and by omitting the species (see Introduction) identified as unsuitable for monitoring by the current method, because detection rates were too low.
Despite the methodological limitations, the indices presented here are considered suitable for monitoring long-term trends in bird populations. The monitoring programme would benefit from certain modifications, but given the long-term increase for all but one species, even when controlling for several factors, our results are sufficiently robust to indicate no cause for concern in the near future over the status of Aldabra’s landbirds.
Supplementary Material
The supplementary materials for this article can be found at journals.cambridge.org/bci
Acknowledgements
This research was carried out by the Seychelles Islands Foundation (SIF) as part of the national project “Strengthening Seychelles’ Protected Area system through NGO management modalities” funded by the Global Environment Facility (GEF Project ID # 3925) and implemented by the Seychelles Department of Environment. As the dataset involved a large number of contributing staff we cannot mention everyone by name but we would like to thank all SIF Aldabra staff and volunteers for their assistance in the landbird monitoring over the years, particularly the Aldabra Research Officers (Anna Liljevik, Rainer von Brandis, Bruno Bautil, Pierre Pistorius, Gavin Hellstroem, Naomi Doak, Jock Currie) and Head Office staff for logistical and administrative support. Specifically, we thank Richard Baxter for providing vegetation classification data and Martijn Hammers for advice on statistics.









