INTRODUCTION
People who inject drugs (PWID) are at risk of injection site infections and injuries. These are a major problem in people who inject psychoactive drugs, such as, heroin or cocaine [Reference Hope1, Reference Hope and Cook2]. However, these have rarely been studied in people who inject image- and performance-enhancing drugs (IPEDs) [Reference Evans-Brown3, Reference McVeigh, Evans-Brown and Bellis4]. Illicit drugs that are used to change image and enhance performance range from those used in body-building, such as anabolic-androgenic steroids (AAS) [Reference Evans-Brown3, Reference Evans-Brown and McVeigh5] to tanning drugs, such as ‘melanotan II’ [Reference Evans-Brown6]. IPEDs are taken both orally and by injection, and many users of these substances take a number of different drugs for the purpose of enhancement, together with ancillary drugs that are used in an attempt to prevent, minimize or treat side-effects [Reference Evans-Brown3, 7, Reference Llewellyn8].
In the UK, attendances at needle-and-syringe programmes (NSPs) by people who inject IPEDs have risen substantially over the last decade [7]. There has also been increasing concern about the harms associated with the use of IPEDs in the UK and elsewhere [Reference Evans-Brown3, 7, Reference Iversen9–Reference Castillo and Comstock12]. Injecting risk behaviours among IPED injectors – principally those who inject AAS – have been assessed [7]. In the UK studies, reported lifetime sharing of injecting equipment ranged from 0·3% to 20% and the sharing of drug vials from 2·4% to 23%, [13] with similar levels of sharing reported elsewhere [Reference Iversen9, Reference Larance10]. Studies have also found that IPED users also report using psychoactive drugs, particularly stimulants, although the concomitant injection of psychoactive drugs is rare [Reference Iversen9, Reference Larance10, Reference Korkia and Stimson14–Reference Ip16].
A range of health problems, including bacterial [Reference Marquis and Maffulli17–Reference Evans20] and bloodborne viral (BBV) [Reference Iversen9, Reference Crampin21–Reference Rich24] infections, have been reported in IPED injectors. A small number of studies have looked at the prevalence of BBV infections in IPED injectors. These studies mostly found the prevalence of BBV infections to be lower than in injectors of psychoactive drugs, but higher than in the general population [Reference Iversen9, Reference Crampin21, Reference Day22, Reference Hope25]. Only one small Australian study of 60 IPED injectors has looked at the extent of other injection-related health problems, and it found that 6% of those surveyed reported ever having an abscess [Reference Larance10].
Injection site infections and injuries have been more widely studied in those who inject psychoactive drugs; with bacterial infections causing problems that range from localized skin and soft-tissue infections to severe systemic illnesses [Reference Hope and Cook2]. Between a quarter and a third of those who inject psychoactive drugs report having had a recent injection site infection or injury [Reference Hope1, Reference Hope and Cook2]. Of those injecting psychoactive drugs these are typically more common in older people, women, the homeless, those with other health problems (such as HIV), those who inject stimulants, those who inject more frequently, and those with poor injection practice or hygiene [Reference Hope1, Reference Hope and Cook2]. However, both the extent of, and risks for, injection site infections and injuries in IPED injectors are likely to be different to those in people who inject psychoactive drugs. First, the effects of psychoactive drugs on the user's behaviour can result in disinhibition and compulsive usage [Reference Simmonds and Coomber26]. Second, IPEDs are usually injected less frequently than psychoactive drugs; and finally, there are differences in injecting practice as IPEDs are only injected subcutaneously or intramuscularly and usually require much less preparation than psychoactive drugs [Reference Evans-Brown3, 7, Reference Llewellyn8, 13, Reference Hope25]. In addition, unlike the commonly injected psychoactive drugs – heroin, amphetamines and cocaine/crack are solids or powders that are dissolved by heating with water or an acidic solution – many IPEDs are liquids that can be contaminated with bacteria during illicit manufacture or distribution [Reference McVeigh, Evans-Brown and Bellis4].
In response to the increasing concerns about IPED use in the UK, a survey of IPED injectors was undertaken during 2010 and 2011 [Reference Hope25]. The aim of this survey – one of the largest of IPED injectors so far undertaken – was to describe the patterns of drug use, the levels of risk behaviours, and the prevalence of infections in IPED injectors [Reference Hope25]. This paper addresses three questions: (a) what is the frequency of self-reported symptoms of injection site injuries or infections in people who inject IPEDs?; (b) what factors are associated with these symptoms?; and (c) to what extent are healthcare services used in response to these symptoms?
METHODS
This survey was undertaken as part of an ongoing annual unlinked anonymous bio-behavioural surveillance study during 2010 and 2011. Details of this survey of IPED injectors, and of the annual surveillance study, have been described previously [Reference Hope25, Reference Hope27]. Briefly, collaborating agencies providing services to PWID (e.g. NSPs and addiction treatment) at sentinel locations throughout England and Wales invite clients who have ever injected drugs to participate in the surveillance study. The sentinel sites are selected so as to reflect both the geographical distribution and range of services offered to PWID. Those who consent to participate provide a biological sample and self-complete a brief questionnaire focused on psychoactive drug use [Reference Hope27]. The surveillance study has multi-site ethics approval. This survey of IPED injectors recruited participants through 19 of the services collaborating in the surveillance study that provided NSPs [Reference Hope25]. IPED injectors in contact with these collaborating services were asked to participate in the survey either when they were visiting a participating fixed site NSP or when they were in contact with staff undertaking outreach work. Those who agreed to participate in this unlinked anonymous survey provided an oral-fluid sample and self-completed a short questionnaire focused on IPED use (types of drug used and routes of administration), related behaviours (injecting practices and sexual behaviours) and health service use. The participants were offered a small acknowledgment in the form of retail vouchers.
The oral-fluid specimens were collected using the OraSure™ device (OraSure Technologies Inc., USA) and were tested for antibodies to HIV (anti-2HIV), hepatitis C virus (anti-HCV) and the hepatitis B core antigen (anti-HBc). The laboratory methods used have been described previously [Reference Hope25]. The anti-HCV test had a sensitivity of 92% and specificity of 99%, and for anti-HBc estimated sensitivity is 75% and specificity 99%.
Women (n = 5), men who reported sex with men (n = 13) and those who reported ever injecting psychoactive drugs (n = 19) were excluded due to small numbers and because these groups may have different risks. Gender has been associated with injection site infections and injuries in people who inject psychoactive drugs [Reference Hope and Cook2]. Men who report sex with men may have different reasons for using IPEDs to heterosexual men (e.g. use of AAS to counteract effects of HIV infection) [Reference Bolding, Sherr and Elford15, Reference Hope25]. Therefore, the participants included in the analyses here were 366 heterosexual male IPED injectors.
Descriptive analyses were undertaken first, then bivariate associations (P < 0·05) between outcomes variables (ever having redness, tenderness and swelling or an abscess, sore or open wound at an injection site) and covariates (age, drug use, sexual practice, and health service use) were examined using Fisher's exact test (when expected cell frequencies were <5) and Pearson's χ 2 test. Where possible associations were found (P < 0·10) these were further examined via logistic regression models using the forward stepwise procedure to select variables, with selection based on the likelihood ratio test (P < 0·05). All analyses were undertaken using SPSS v. 19 (SPSS Inc., USA).
RESULTS
Of the 366 male IPED injectors, 28% (102) were aged <25 years, with 26% (90) aged ⩾35 years; however, 13% (47) did not report their age (of those reporting age, the median was 28 years, quartiles 23 and 35 years). For 248 it was possible to calculate time since first IPED use: of these 53% had been using IPEDs for <5 years (median 4 years, quartiles 1 and 9 years). Overall, 16% (57) had ever been in prison. Testing of the oral-fluid samples indicated that 0·82% (3) had anti-HIV, 4·8% (adjusting for sensitivity, 4·4%, 16) had anti-HCV and 8·0% (adjusting for sensitivity, 6·0%, 22) had anti-HBc [Reference Hope25].
The participants reported injecting a range of IPEDs during the preceding year: anabolic steroids were reported by 85% (312), growth hormone by 32% (116), human chorionic gonadotropin (hCG) by 16% (58), ‘melanotan I/II’ by 8·5% (31), and insulin (as an IPED) by 4·6% (17). One in twenty (4·9%, 18) had injected a less commonly used IPED (including erythropoietin, insulin-like growth factor-1 and nalbuphine hydrochloride). During the preceding year, 87% (318) had injected intramuscularly and 40% (145) subcutaneously, with 18% (65) reporting that they were usually injected by someone else [with 63 (97%) of these reporting they injected intramuscularly and 20 (31%) subcutaneously].
Symptoms of injection site infections and injuries
Ever having redness, tenderness and swelling at an injection site was reported by 42% (155) of the participants. Of these, 133 reported having had this symptom during the preceding year (36% of the whole sample), with 61% (81) reporting 2–9 episodes and 7·5% (10) ⩾10 episodes. Ever having an abscess, sore or open wound at injection site was reported by 6·8% (25), with 37·5% (9/24) of these men reporting that they had ever had ⩾2 episodes.
The associations, both bivariate and multivariate, between covariates and ever having redness, tenderness and swelling at an injection site are given in Table 1. In the multivariate analysis ever having redness, tenderness and swelling was associated with having used IPEDs for ⩾5 years, ever having attended a NSP site, having injected hCG in the preceding year, and reporting ever sharing a needle, syringe or vial.
Table 1. Factors associated with ever having had redness, tenderness and swelling at an injection site
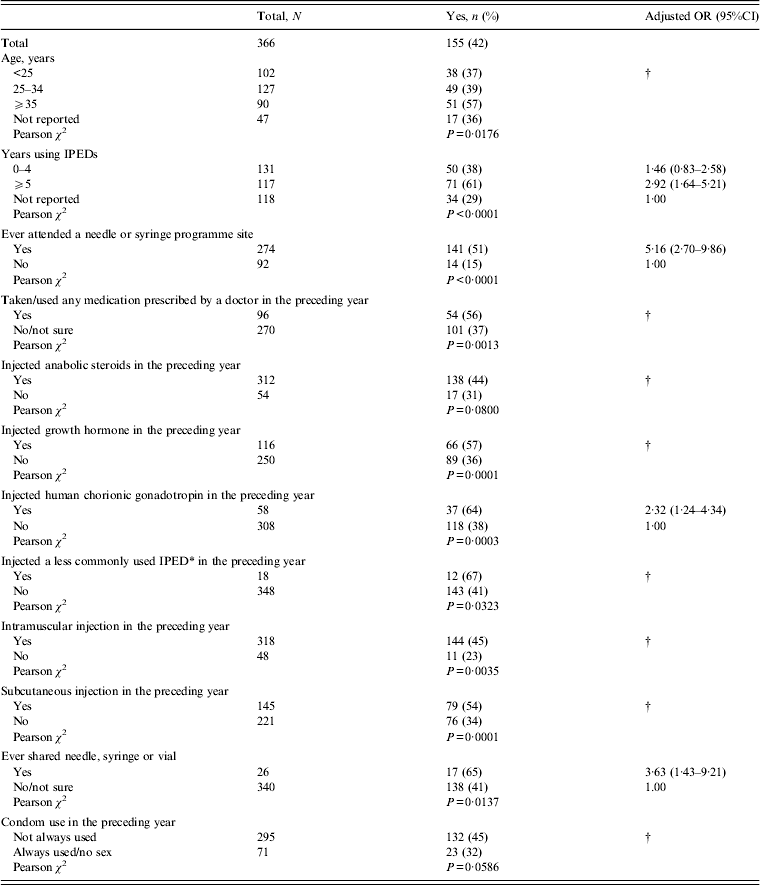
OR, Odds ratio; CI, confidence interval; IPED, image- and performance-enhancing drug.
No associations were found with: having ever been in prison; having seen a General Practitioner in the preceding year about their health; having sought advice from a sexual health clinic in the preceding year; having had a blood test for hepatitis C; having had a blood test for HIV; injecting ‘melanotan I or II’ in the preceding year; snorting cocaine in the preceding year; snorting or swallowing amphetamine in the preceding year; who usually injected them during the preceding year; oral fluid sample anti-HIV test result; oral fluid sample anti-HBc test result; and oral fluid sample anti-HCV test result.
* Drugs used included erythropoietin, insulin-like growth factor-1 and nalbuphine hydrochloride.
† Not in final model.
The bivariate and multivariate associations between covariates and ever having abscess, sore or open wound at an injection site are given in Table 2. In the multivariate analysis ever having had an abscess, sore or open wound was associated with not having sex in the preceding year, having ever attended a NSP site, having received advice from an emergency clinic in the preceding year, not having taken up the vaccine against hepatitis B, and being HIV antibody positive.
Table 2. Factors associated with ever having had an abscess, sore or open wound at an injection site
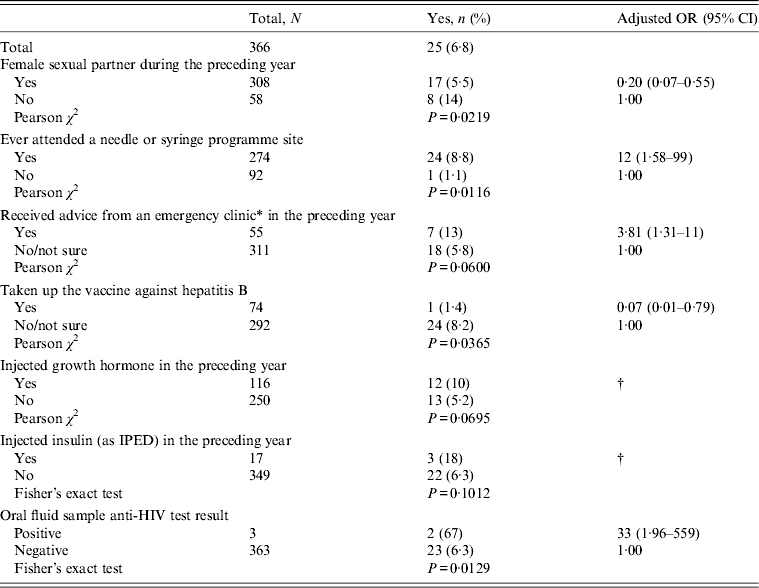
OR, Odds ratio; CI, confidence interval.
No associations were found with: age in years; having ever been in prison; having seen a General Practitioner in the preceding year about their health; having taken/used prescribed medication in preceding year; having sought advice from a sexual health clinic in the preceding year; having had a blood test for hepatitis C; having had a blood test for HIV; number of years since first used an image- and performance-enhancing drug (IPED); injecting anabolic steroids in the preceding year; injecting human chorionic gonadotropin in the preceding year; injecting ‘melanotan I or II’ in the preceding year; injecting a less commonly used IPED in the preceding year; snorting cocaine in the preceding year; snorting or swallowing amphetamine in the preceding year; who usually injected them during the preceding year; intramuscular injection in the preceding year; subcutaneous injection in the preceding year; ever sharing a needle, syringe or vial; unprotected sex in the preceding year; oral fluid sample anti-HBc test result; and oral fluid sample anti-HCV test result.
* Accident and Emergency department or Walk-in clinic.
† Not in final model.
Healthcare usage
Overall, 43% (159) had seen a General Practitioner (GP) in the preceding year about their health, with 14% (22/158) reporting a visit to a GP that was related to their IPED use. Of those who had seen a GP, 41% (64/158) said that the GP knew about their IPED use. During the preceding year, 15% (55) had received advice from an emergency clinic – i.e. either an Accident & Emergency department or a Walk-in clinic – and 41% (21/51) had done so more than once. Overall, 15% (56) had sought advice from a sexual health clinic in the preceding year (28%, 14/50, of these had done so more than once). Three-quarters (75%, 274) reported that they had ever visited a NSP site (reflecting the recruitment of many of the participants through such services).
A quarter (26%, 96) had taken/used prescribed medication in the preceding year, with 5·2% (5/95) reporting this was related to their IPED use. Of those who had taken prescribed medication 64% (61/96) reported doing so while using IPEDs. Overall, 20% (74) had taken up the vaccine against hepatitis B, 18% (65) had ever had a diagnostic test for hepatitis C, and 28% a test for HIV.
One sixth (17%, 27) of those who reported having redness, tenderness and swelling at an injection site indicated that they had ever sought treatment for this symptom (Table 3). Of those who had sought treatment this had most often been done through a GP (59%), while 48% had used an emergency clinic or attempted to self-treatment (Table 3). The majority (76%, 19) of those having an abscess, sore or open wound at an injection site had ever sought treatment for this symptom (Table 3). Of those who had sought treatment, 47% had attended an emergency clinic and 32% had used a GP, with 26% attempting self-treatment (Table 3).
Table 3. Seeking treatment for either, redness, tenderness and swelling or an abscess, sore or open wound at an injection site

DISCUSSION
Injection site problems were common with over one-third of male IPED injectors reporting redness, tenderness and swelling at an injection site in the past year – with many of these men having multiple episodes. Overall, one-sixth had ever sought treatment for redness, tenderness and swelling, and more than 1/15 reported having ever had an abscess, sore or open wound at an injection site. Although substantial these levels are lower than those found in people who inject psychoactive drugs. In the UK around half those who inject psychoactive drugs report having had redness, tenderness and swelling and around one third an abscess, sore or open wound at an injection site in the past year [Reference Hope1, Reference Hope and Cook2]. This difference may in part reflect the fact that people who inject psychoactive drugs probably inject much more frequently than those using IPEDs.
The association between having an abscess, sore or open wound at an injection site and HIV positivity probably reflects the greater vulnerability of those with HIV to injection-related bacterial infections [Reference Hope and Cook2]. The associations with ever having attended a NSP and having obtained advice from an emergency clinic in the preceding year may reflect healthcare-seeking behaviour related to this symptom. The associations with not having sex in the preceding year, and not taking up the vaccine against hepatitis B, both need further examination, but these might be markers for other factors not examined here, such as the reasons for IPED use or to the side-effects of the IPEDs being used [Reference Evans-Brown3, Reference McVeigh, Evans-Brown and Bellis4].
The associations between ever having redness, tenderness and swelling and having used IPEDs for ⩾5 years, having injected hCG in the preceding year, and reporting ever sharing a needle, syringe or vial suggest that this symptom may in part be related to certain patterns of IPED use. For example, hCG is injected subcutaneously and those who have been using IPEDs for longer might be using a wider range of substances, using more frequently, or taking larger doses [Reference Evans-Brown3, Reference Evans-Brown and McVeigh5]. Again this needs further investigation. The association with ever having attended a NSP may be related to having sought healthcare or advice about this symptom.
The development of these symptoms may in part be due to the effects of the IPEDs used – particularly of AAS – on the user's physiology [Reference Evans-Brown3, Reference McVeigh, Evans-Brown and Bellis4]. The use of AAS has been reported to affect the immune response, and in vitro studies indicate that supra-physiological doses of some AAS may be immunosuppressive [Reference Marshall-Gradisnik, Green and Weatherby28]. AAS can also cause cutaneous changes resulting in acne, rosacea, epidermoid cysts, seborrhoeic dermatitis, and oily skin [Reference Walker and Adams29]. These physiological changes caused by AAS use may therefore increase the user's risk of developing injection site infections. In addition, counterfeit or sub-standard AAS have long been recognized as an issue in IPED users in the UK and elsewhere [Reference Llewellyn8, Reference Korkia and Stimson14, Reference Duchaine30–Reference Evans-Brown, Kimergård and McVeigh32]. These include illicitly produced AAS, manufactured without any guarantee of quality or safety, that have the potential to result in infection and injury, for example due to bacterial contamination, even when hygienic and appropriate injecting techniques are used.
Only a minority (1/6) of those reporting redness, tenderness and swelling had either sought treatment or treated that symptom. This might reflect most episodes of this symptom being minor, or that this symptom may, for some at least, represent an accepted side-effect of IPED injection. Three quarters of those reporting an abscess had sought treatment for it. Many of those reporting treatment for these symptoms, had treated themselves. This may be indicative of a reluctance to access health services, possibly related to concerns about disclosing their drug use [Reference Marquis and Maffulli17, Reference Dunn19, Reference Simmonds and Coomber26, Reference Burton33]. The proportion of those reporting redness, tenderness and swelling seeking treatment for this symptom was lower than for those injecting psychoactive drugs; however, the proportion of those with an abscess seeking treatment was similar [Reference Hope1]. Furthermore, IPED injectors accessed the same health services as those used by people injecting psychoactive drugs [Reference Hope1].
The findings from this study indicate that those providing services that may be accessed by men who inject IPEDs – such as NSPs, GPs and emergency clinics – should be alert to the risks and health consequences associated with the use of these drugs. In particular, they need to be aware of the range of drugs that may be used, the associated injecting practices – as these differ from those of injectors of psychoactive drugs – and the harms that can result. Considering the levels of injection site infections and injuries found here, specialist services for PWID need to engage with people who inject IPEDs so as to ensure that they have access to appropriate injecting equipment and targeted harm reduction advice, in particular, advice on safer IPED use and injecting practice. This should include advice on the risk of infections, and other hazards, that can result from using illicit products which may be sub-standard, unsterile or otherwise contaminated.
It is important to consider the limitations of this study. The representativeness of those recruited is impossible to measure, as the illicit nature, marginalization, and comparative rarity of injecting drug use, all impeded the construction of a sampling frame for this population. This study used an established methodology for recruiting PWID through health services [Reference Morrison, Elliott and Gruer34]; however, the robustness of this approach for people who inject IPEDs is not known and cannot currently be assessed due to the limited information on the nature and size of this group [Reference Evans-Brown3, 7]. The findings here also rely on self-report – although the reliability of these has not been assessed in IPED users, studies have shown concordance between self-reported symptoms and clinical diagnosis in psychoactive drug users [Reference Morrison, Elliott and Gruer34]. Considering these limitations caution is advised when attempting to generalize the findings to the wider population of IPED injectors.
This study can only provide a partial understanding of the risks associated with injection site infections and injuries as, because of the varied nature of injection and drug use practice among users of IPEDs, only limited data was collected on injection technique. Previous studies [Reference Larance10], together with information from genre publications and online forums indicate a range of IPED injecting practices, and that some specific techniques and behaviours may be associated with an increased risk of injection site problems. One common technique in some groups of AAS users is ‘spot injections’ that is, injection into small muscle groups, such as the biceps, triceps and calf muscles [Reference Larance10]. This is done for cosmetic purposes in the belief that this will stimulate localized muscle growth [Reference Llewellyn8]. These smaller muscles are more difficult to navigate, require some anatomical knowledge and are more prone to complications [Reference Nicoll and Hesby35]. Case reports have identified compartment syndrome and localized rhabdomyolysis as a result of inappropriate injection techniques [Reference Farkash, Shabshin and Pritsch (Perry)36, Reference Akhavani, Pacifico and Kang37]. There is thus a need to build on the findings of this study through more detailed investigations to examine the injecting techniques that are being used, the associated patterns of IPED use and the resulting harms.
Our findings suggest that injection site infections and injuries are a common occurrence in men who inject IPEDs. This study recruited participants through services that provide injecting equipment and advice, IPED users not in contact with such services may have a different pattern of risk. Even so, our findings suggest the need for targeted interventions to address drug use patterns, injection practices and the health of men who inject IPEDs. Possible interventions include harm reduction activities to: support hygienic intramuscular and subcutaneous injecting, by promoting hand washing and the swabbing of injection sites; raise awareness about the illicit manufacture of IPEDs and that their contamination is commonplace; and provide advice on safer injection practice and IPED use. Although our current knowledge of this population is limited, particularly of those IPED injectors not in contact with NSP services and outreach workers, evidence from other hidden populations suggests several intervention approaches that could be appropriate additions to the provision of targeted NSPs. In particular, the use of peer educators [Reference Newland and Treloar38] and community mobilization [Reference Rhodes39] may be effective intervention approaches for delivering harm reduction advice to this population. The development and evaluation of such interventions, and the evaluation of targeted NSP provision in line with recently updated guidance [40], should be considered. Further studies recruiting users of IPEDs from a wider range of settings are also needed to more fully examine risk factors and to better assess appropriate response options.
ACKNOWLEDGEMENTS
We are grateful to all of the people who took part in the survey and to the various services across the England and Wales who assisted with their recruitment. We also thank the support staff who worked on the survey and those who undertook the laboratory work. The survey was undertaken by the Health Protection Agency (now part of Public Health England) which is funded through the Department of Health. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.








