INTRODUCTION
Each year, around 25% of the Dutch population visit their General Practitioner (GP) with respiratory symptoms [1]. Part of this group presents with a lower respiratory tract infection (LRTI), which is generally more serious than an upper respiratory infection. A Dutch study showed that patients with community-acquired pneumonia still have an impaired health status 18 months after onset of illness compared to a control population, although these results were attributed more to the effects of age and/or comorbidity than the pneumonia [Reference El2]. Furthermore, several studies have shown that Q fever, an infectious illness which presents with high rates of pneumonia in patients in some countries [Reference Raoult, Marrie and Mege3] (61·5% in The Netherlands [Reference Dijkstra4]), may have a long-term impact on patients’ health [Reference Morroy5–Reference Hickie10]. We found limited information on long-term health status of LRTI patients in general [Reference El2]. We assessed the health status of patients who experienced a LRTI the previous year by using a standard questionnaire. Special attention was paid to Q fever in this study, because of the large outbreak that affected The Netherlands during that period [11]. Since patients with Q fever as well as patients with other causes of LRTI appear to be at risk for long-term impaired health status, including fatigue, we investigated whether a LRTI caused by Q fever is a more severe infection in terms of health status at ~15 months after onset of illness than other LRTIs.
METHODS
Design
In a cross-sectional cohort study, patients presenting with a LRTI to their GP in 2009 were included, and subsequently their health status was assessed at ~15 months after onset of illness.
Study site
GP practices (n = 14) in the provinces of Northern Brabant and Gelderland, located in or around the epicentre of the Q fever outbreak in The Netherlands, registered patients with a LRTI.
Study population
Patients with a LRTI, as diagnosed by their GP, were included in the study. Diagnosis was based on clinical symptoms. Patients were categorized into one of the following International Classification of Primary Care (ICPC) groups: R78 acute bronchitis, R80 influenza, R81 pneumonia and R83 other lower respiratory tract infections. Patients aged <18 and >75 years were excluded since the proportion of Q fever infections compared to other infections is limited for these age groups. The inclusion period was from 1 May to 30 September 2009, to exclude a high proportion of pathogens specific for the winter period. All included patients were serologically tested for Q fever in one out of two hospital laboratories as part of regular care. Diagnostic tests were polymerase chain reaction (PCR), immunofluorescence assay (IFA) and complement fixation assay (CFA). Patients were diagnosed as either Q fever positive or Q fever negative. Regular care for Q fever-positive patients also included serological follow-up to diagnose potential cases of chronic Q fever, but these results were not included in our study. Of the 194 registered LRTI patients who were tested for Q fever in 2009, 19 patients could not be contacted, two patients died and six patients moved to a GP practice not included in the study area. This left a total of 167 patients that were invited to participate.
Data collection
Information on hospitalization of patients during the acute phase of the disease was obtained through their GPs. Between July and September 2010, patients received a health status questionnaire with a consent form from their GP. If the patient did not return the questionnaire within 4 weeks, a reminder was sent by the GP.
Health status questionnaire
Health status was assessed using the Nijmegen Clinical Screening Instrument (NCSI). The NCSI is a validated instrument and measures health status on eight subdomains of three domains: ‘Symptoms’, ‘Functional impairment’ and ‘Quality of life’. The NCSI consists of a battery of instruments (Table 1) and provides a valid and detailed picture of a patients’ health status [Reference Peters12]. It allows a description of health status at the individual level (as a normal, mild or severe score is available for each subdomain). In addition, the questionnaire contained questions on personal characteristics (gender, age, smoking behaviour) and comorbidity.
Table 1. Nijmegen Clinical Screening Instrument subdomains
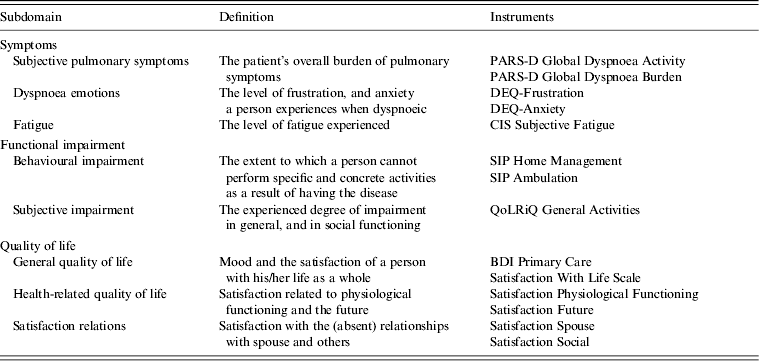
PARS-D, Physical Activity Rating Scale – Dyspnoea; DEQ, Dyspnoea Emotions Questionnaire; CIS, Checklist Individual Strength; SIP, Sickness Impact Profile; QoLRiQ, Quality of Life for Respiratory Illness Questionnaire; BDI, Beck Depression Inventory.
Statistical analysis
SPSS for Windows v. 20 (IBM SPSS Statistics, USA) was used for data entry and analyses of the data. A value of P < 0·05 was considered as statistically significant. All identifiers were removed and data were analysed anonymously. The baseline data of 2009 (from the GP registration of LRTI patients) enabled us to compare responders and non-responders with regard to gender, age, ICPC, hospitalization and Q fever status. χ 2 tests and an unpaired t test were used for comparison of characteristics between patients who tested positive for Q fever vs. patients who tested negative for Q fever.
Scores of all eight subdomains of the NCSI were calculated and the proportion of patients with normal, mild and severe scores on the different subdomains were determined, as described in a study by Peters et al. [Reference Peters12].
Differences in NCSI subdomain scores between the group of Q fever-positive and Q fever-negative LRTI patients were analysed using a multivariate model for each subdomain, with correction for relevant confounding characteristics, i.e. gender, age, smoking behaviour, ICPC and comorbidity. ICPC was dichotomized into two items; pneumonia (R81) and other LRTI (an aggregation of R78, R80 and R83). Comorbidity was also dichotomized into two items due to small numbers: no comorbidity vs. one or more underlying diseases (e.g. heart or vascular disease, chronic disease, cancer, immune disorder, diabetes, lung disease, depression).
RESULTS
Eighty-two patients returned the questionnaire, resulting in a response rate of 49%. Patients completed the questionnaire 10–19 months after initial infection in 2009, with a mean response time of 15 months. There was no significant difference in gender, age and hospitalization between responders and non-responders (data not shown). Responders more often had pneumonia as an ICPC classification (65% vs. 42%, P = 0·004) and more often tested positive for Q fever in 2009 (61% vs. 45%, P = 0·035) compared to non-responders.
Characteristics of the study population
Of the responders, 50 (61%) patients tested positive for a Q fever infection (Table 2). Significantly more Q fever-positive patients were diagnosed with pneumonia compared to Q fever-negative patients (76% vs. 47%, P = 0·004). Q fever-positive patients were younger (mean age 48·1 years) than Q fever-negative patients (mean age 57·2 years), although the difference was not significant. There were no significant differences between the two groups for hospitalization at baseline, gender, smoking behaviour and comorbidity.
Table 2. Comparison of the characteristics of the study groups, consisting of Q fever-positive and Q fever-negative LRTI patients
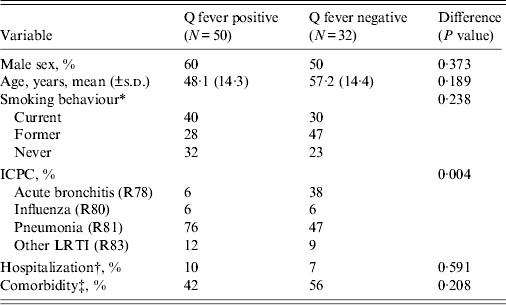
LRTI, Lower respiratory tract infection; ICPC, International Classification of Primary Care.
* There were two missing values for smoking behaviour.
† Hospitalization was measured at baseline and there were three missing values.
‡ Comorbidities consist of (among others) heart or vascular disease, chronic disease, cancer, immune disorder, diabetes, lung disease, depression.
Health status
Health status of a large proportion of the patients within each group was severely affected at ~15 months after onset of illness as measured by the NCSI, ranging from 12% on the subdomains ‘satisfaction relations’ and ‘behavioural impairment’ to 64% on the subdomain ‘fatigue’ (Fig. 1). Within the Q fever-positive LRTI group, ‘general quality of life’ (40%) and ‘fatigue’ (40%) were the most severely affected subdomains, while most severely affected subdomains of the Q fever-negative LRTI group were ‘fatigue’ (64%) and ‘subjective pulmonary symptoms’ (35%). The proportions of patients who were severely affected on more than one subdomain ~15 months after onset of illness were 40% and 56% for the Q fever-positive and Q fever-negative LRTI patients, respectively.
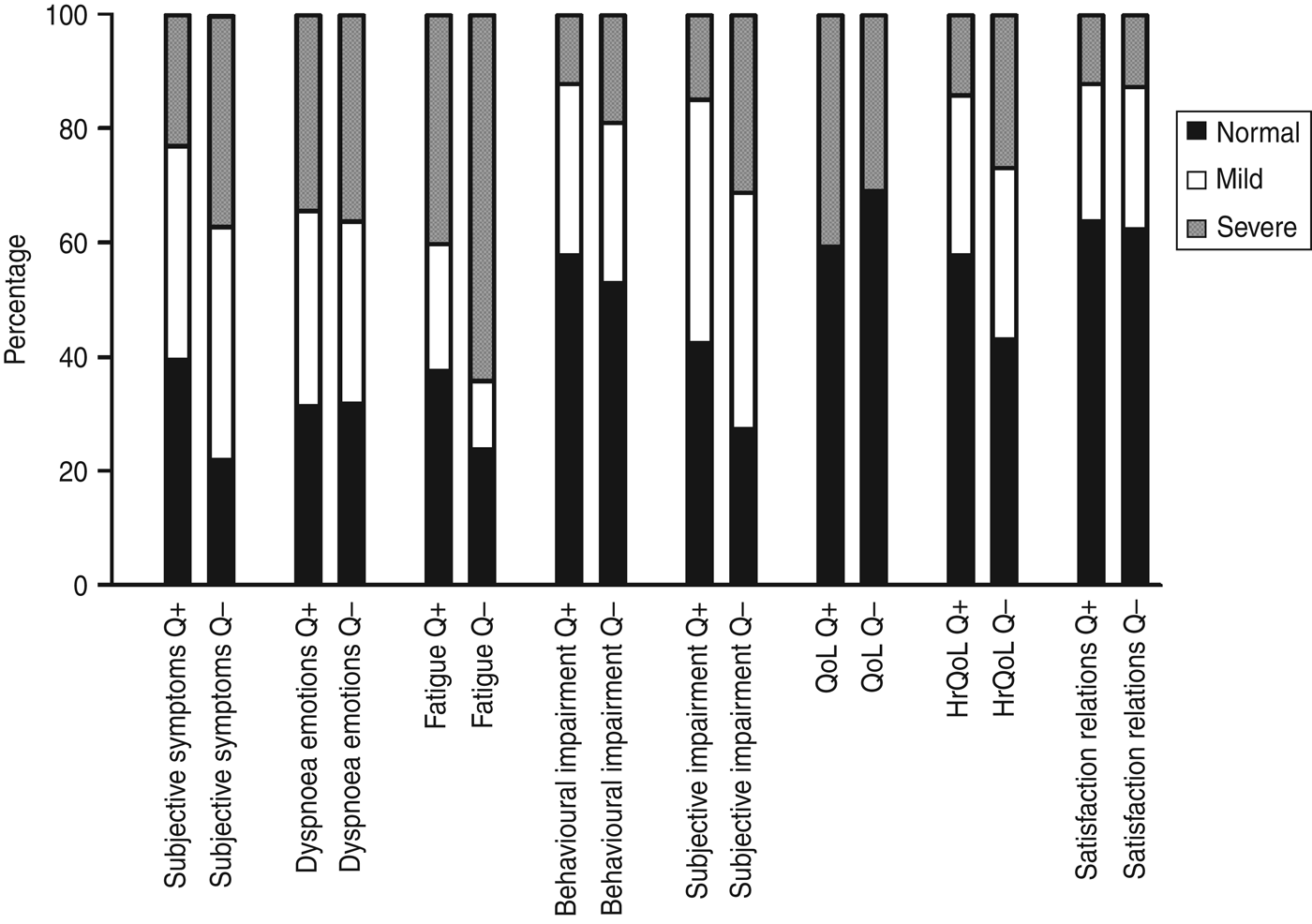
Fig. 1. Proportion of patients with normal/mild/severe scores on the different subdomains of the Nijmegen Clinical Screening Instrument at ~15 months after lower respiratory tract infection, presented for Q fever-positive (Q+) and Q fever-negative (Q–) patients. QoL, Quality of life; HrQoL, health-related quality of life.
Health status scores between Q fever-positive and Q fever-negative LRTI patients were compared at ~15 months after initial illness. Q fever-negative patients scored significantly worse for the subdomain ‘subjective pulmonary symptoms’ after correcting for the confounders gender, age, smoking behaviour, pneumonia and comorbidity (2·62, P = 0·048) (Table 3).
Table 3. Linear regression models presenting the NCSI scores for each subdomain ~15 months after LRTI for Q fever-positive and Q fever-negative LRTI patients corrected for gender, age, smoking behaviour, ICPC (pneumonia or other) and comorbidity (yes or no). Q fever-positive patients are the reference group
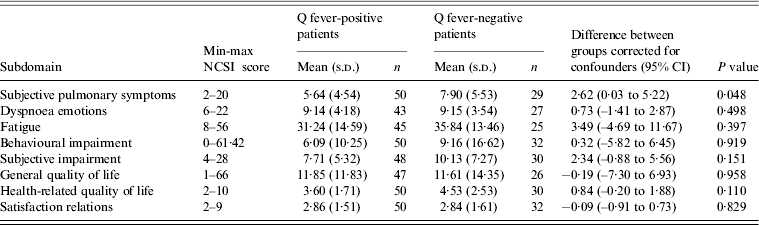
NCSI, Nijmegen Clinical Screening Instrument; LRTI, Lower respiratory tract infection; ICPC, International Classification of Primary Care; CI, confidence interval.
DISCUSSION
This study demonstrates that a large group of GP-registered LRTI patients was affected on one or more aspects of health status ~15 months after LRTI, especially on ‘fatigue’, ‘general quality of life’ and ‘subjective pulmonary symptoms’. These long-term symptoms have also been described in a study by El Moussaoui et al. in community-acquired pneumonia patients with an impaired health status 18 months after their initial illness, especially in patients with a comorbidity [Reference El2]. Long-term symptoms and an impaired health status were also seen in patients with Legionnaires’ disease, for which most patients experience pneumonia during the acute phase of the disease [Reference Lettinga13].
There was no significant difference in health status scores at ~15 months between LRTI patients who were diagnosed with Q fever compared to patients who did not have Q fever, except for the subdomain ‘subjective pulmonary symptoms’. The Q fever-negative group experienced significantly more subjective symptoms (overall burden of pulmonary symptoms) than the Q fever-positive group, although we cannot explain why this group had more symptoms. A previous study in The Netherlands identified having Q fever as well as pneumonia as risk factors for a long-term impaired health status [Reference Morroy5], which is why one would expect a larger impact on health in the Q fever-positive group of LRTI patients. The main outcome of our study is, however, that long-term health status of Q fever-positive and Q fever-negative LRTI patients was very similar.
Results concerning Q fever patients within this study are comparable to previous Dutch Q fever studies, even though this study only considers Q fever patients with a LRTI (in contrast to other studies, where all Q fever patients are considered). ‘Fatigue’ and ‘general quality of life’ were the subdomains with the highest proportions of severe scores; these results were also found in the other Dutch studies [Reference Morroy5, Reference Limonard6]. Forty per cent of the Q fever patients showed severe fatigue at ~15 months after their initial illness, which is similar to the 44% and 52% from the other studies as well. Studies outside The Netherlands also showed fatigue as one of the main long-term health problems for Q fever patients [Reference Ayres7–Reference Hatchette9]. However, it has been shown that over 30% of the general population suffer from chronic fatigue [Reference van't Leven14, Reference Kocalevent15], which raises uncertainty about the proportion of fatigue in patients that can be attributed to Q fever.
Strengths and limitations of the study
Despite the fact that reminders were sent and that patients received the questionnaire from their own GP, the response rate was relatively low (49%). The fact that responders were more often Q fever positive may have been due to the fact that Q fever and its burden of disease received a great deal of media attention during the outbreak. Q fever patients may therefore have deemed it more important to complete a questionnaire on their health status, despite the fact that the letter and questionnaire that were sent to patients did not contain the word ‘Q fever’. The low response rate may have resulted in a relatively high proportion of study participants with an impaired health status, especially in the Q fever-negative group (patients with symptoms are considered more eager to participate in studies), indicating that our results might show an overrepresentation of their health impact.
Patients were tested for Q fever by two different laboratories, using different diagnostic methods. The most frequently used laboratory tests in Q fever-positive patients were the IFA (50%) and PCR (43%). However, all tests used are considered suitable serodiagnostic assays to diagnose acute Q fever [Reference Schneeberger16, Reference Herremans17].
A potential limitation of our study was that we did not further diagnose the microbiological cause of illness of the Q fever-negative LRTI patients. A study conducted in The Netherlands showed that a wide variety of pathogens is present in patients with acute respiratory tract infections [Reference van Gageldonk-Lafeber18], which indicates that it is often difficult to establish the source of an infection in this population. Moreover, Marrie et al. were unable to find any difference in disease recovery at 30 days after onset of illness in patients with atypical pneumonia with unknown microbiological cause and patients with atypical pneumonia due to a pathogen from a series of underlying agents [Reference Marrie19]. We do not therefore feel this disproves the overall findings and conclusions.
More generally, studies on the health impact of infectious diseases have demonstrated that long-term recovery in patients with varying microbiological diseases, such as Epstein–Barr virus, enteroviruses and Coxiella burnetii, all experience long-term fatigue [Reference Hickie10, Reference Devanur and Kerr20], and that post-infective fatigue syndrome is largely predicted by severity of the acute illness rather than by microbiological factors [Reference Hickie10]. The observation in our study that Q fever-positive as well as Q fever-negative LRTI patients showed a long-term impaired health status is in line with these studies.
CONCLUSIONS
This study showed that a large group of LRTI patients was affected on more than one aspect of health status at ~15 months after LRTI. We have demonstrated that there is little difference in long-term health status between Q fever-positive and Q fever-negative LRTI patients. GPs should be aware of long-term health problems in LRTI patients, not only those that are Q fever positive but also those that are Q fever negative.
ACKNOWLEDGEMENTS
We thank all the GPs from the NUHP (Network of GP practices affiliated to Radboud University Medical Centre) who participated in the study, and Clementine Wijkmans from the ‘GGD Hart voor Brabant’ for the initial idea and preparations of the study. The study was funded by Robuust, a regional supporting organization for primary care in the South of The Netherlands and the Provinciale Raad Gezondheid (county council of health) in the province of Northern Brabant.
DECLARATION OF INTEREST
None.









