The red panda Ailurus fulgens, endemic to the Himalayan areas of Bhutan, China, India, Myanmar and Nepal (Roberts & Gittleman, Reference Roberts and Gittleman1984), inhabits evergreen, deciduous, and mixed evergreen–deciduous forests with dense understories (Yonzon & Hunter, Reference Yonzon and Hunter1991) at elevations of 1,500–4,800 m (Choudhury, Reference Choudhury2001). Red pandas require fallen logs for resting and feeding, bamboo or fruits for forage, and open water (Wei et al., Reference Wei, Feng, Wang and Hu2000). The red panda is categorized as Vulnerable on the IUCN Red List (Wang et al., Reference Wang, Choudhury, Yonzon, Wozencraft and Than2008) and in Nepal is a protected priority species (Jnawali et al., Reference Jnawali, Baral, Lee, Acharya, Upadhyay and Pandey2011) under the National Parks and Wild Conservation Act 1973. Range-wide threats include killing by poachers and dogs, and habitat loss (Wei et al., Reference Wei, Feng, Wang and Hu1999; Williams, Reference Williams2003). Livestock grazing and collecting livestock feed (Sharma & Belant, Reference Sharma and Belant2010) may further reduce the quality of habitat for the red panda. Understanding habitat selection by the red panda, especially any effects of anthropogenic activities on such selection, is important for the conservation of the species.
Livestock grazing is considered a major threat to wildlife in protected areas in Nepal, including in Langtang National Park, Dhorpatan Hunting Reserve and Koshi Tappu Wildlife Reserve (Berkmueller et al., Reference Berkmueller, Mukherjee and Mishra1990; Yonzon & Hunter, Reference Yonzon and Hunter1991; Sharma & Belant, Reference Sharma and Belant2010), and elsewhere (e.g. Bhutan; Dorji et al., Reference Dorji, Rajaratnam and Vernes2012). Livestock, particularly cattle and buffalo, can damage habitats, compete with native species for food and introduce diseases (Jolles et al., Reference Jolles, Cooper and Levin2005; Schmidt et al., Reference Schmidt, Olsen, Bildsøe, Sluydts and Leirs2005). Consequently, we considered it relevant for red panda conservation generally to assess the effects of livestock use on red panda habitat selection in Rara National Park.
We collected data on presence of livestock sign and red panda faecal pellet groups in Rara National Park (Fig. 1) during May–July in 2011 and 2012. The study area is at altitudes of 2,754–4,097 m and is dominated by blue pine Pinus wallichiana up to 3,200 m, with rhododendron Rhododendron arboreum, black juniper Juniperus indica, west Himalayan spruce Picea smithiana, oak Quercus semecarpifolia, and Himalayan cypress Cupressus torulosa. Mixed forests of blue pine, west Himalayan spruce and fir Abies spectabilis occur over 3,200–3,550 m, and above 3,350 m there is coniferous–broadleaf forest of fir, oak, birch Betula utilis, Indian horse-chestnut Aesculus indica, walnut Juglans regia and Himalayan poplar Populus ciliata. The Park is surrounded by nine Village Development Committees (administrative units within a district), which were declared a buffer zone to increase people's participation in conservation and minimize their conflict with the Park (Budhathoki, Reference Budhathoki2004). The buffer zone and the Park together comprise 304 km2. Livestock grazing occurred in the Park and buffer zone before its establishment in 1976 but is now illegal in the Park but not in the buffer zone. The Park is unfenced.
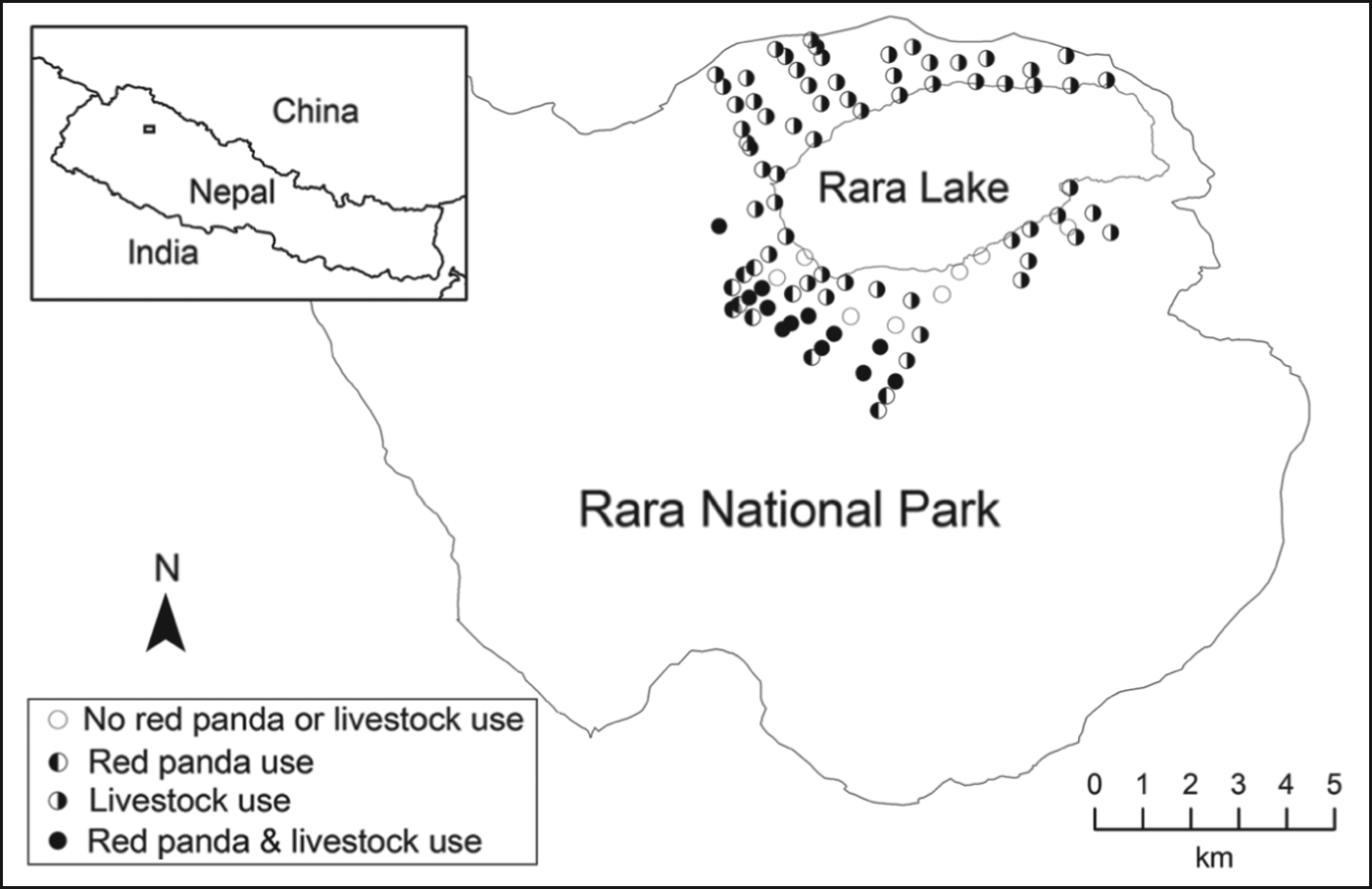
Fig. 1 Distribution of plots (circles) surveyed for signs of the presence of livestock and the red panda Ailurus fulgens in Rara National Park, Nepal, during 2011–2012. The inset indicates the location of the main map in Nepal.
We delineated 25 linear transects at 500-m intervals in the forest around Rara Lake within the Park and established 10 × 10 m plots along each transect. Mean transect length was 1.46 ± SD 0.51 km (range 0.6–2.5 km). Transects started within 50 m of the road that circumscribes Rara Lake and plots were placed at each 100-m increase in elevation. At each plot we recorded aspect and elevation, using a global positioning system, measured distance from the plot centre to the nearest open water, using a measuring tape, and recorded any signs of recent livestock occurrence (livestock dung, tracks and trampling of vegetation), bamboo and red panda faecal pellet groups.
We calculated means and standard errors of continuous variables for plots with and without red panda pellets. We used logistic regression to estimate the effects of bamboo presence, elevation, livestock presence, aspect and distance to the nearest water on the presence of red panda faecal pellet groups. Because red pandas are bamboo specialists (Roberts & Gittleman, Reference Roberts and Gittleman1984), we included bamboo presence in all models and ran all combinations of variables without interactions. Before conducting logistic regression we calculated a correlation matrix of the explanatory variables, to exclude those with |r| > 0.7 in the same model (Libal et al., Reference Libal, Belant, Leopold, Wang and Owen2011). We ranked models using the Akaike information criterion adjusted for small samples (AICc; Burnham & Anderson, Reference Burnham and Anderson2002) and used Akaike model weights to estimate relative strength of evidence for each model. We conducted model averaging using all models, to estimate 95% confidence intervals for each variable, and accepted statistical significance at α < 0.05.
We surveyed a total of 106 plots on 25 transects (14 transects on north-facing and 11 on south-facing slopes). We detected red panda pellet groups in 23 plots (43%) on north-facing slopes at 3,185–3,516 m and none on south-facing slopes. Mean elevation of plots with and without red panda pellet groups was 3,166 ± SE 19.7 m and 3,205 ± SE 22.9 m, respectively. Bamboo (Thamnocalamus spp.) occurred only in plots on north-facing slopes (62%) at 2,985–3,516 m. Mean distances to nearest open water for plots with and without red panda pellet groups were 60.6 ± SE 4.9 m and 99.6 ± SE 11.3 m, respectively; 79% of plots on north-facing slopes were ⩽ 100 m from water. Livestock presence was documented in 81% of plots (64 and 98% of plots on north-facing and south-facing slopes, respectively) at 2,985–3,600 m.
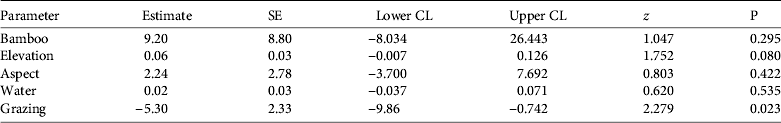
No pairs of variables were highly correlated (|r| < 0.7 for all correlations) and thus all were used for modelling. The best-supported models included bamboo and elevation, followed by the model containing bamboo, elevation and aspect (Table 1). The probability of red panda presence was less in plots with livestock use (Table 2).
Table 1 Logistic regression models describing the occurrence of the red panda Ailurus fulgens in Rara National Park (Fig. 1) during 2011–2012, ranked according to the Akaike information criterion adjusted for small sample size (AICc). Model parameters include Bamboo (bamboo presence), Elevation (m), Aspect (north or south), Water (distance to nearest open water), and Grazing (presence of livestock grazing). K is the number of parameters, ∆AICc is the difference between the AICc value of the best-supported model and successive models, and wi is the Akaike model weight.
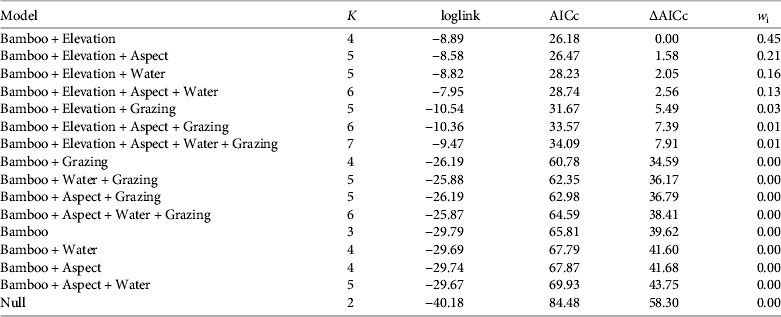
Table 2 Model-averaged parameter estimates and 95% confidence limits (CL) describing red panda occurrence in Rara National Park (Fig. 1) during 2011–2012. Model parameters include Bamboo (bamboo presence), Elevation (m), Aspect (north or south), Water (distance to nearest open water), and Grazing (presence of livestock grazing). Estimates were averaged from all models.
The National Park Regulations (HMG, 1979) stipulate that Park authorities can allow livestock grazing if it is important for management (Heinen & Kattel, Reference Heinen and Kattel1992). Livestock grazing is illegal in Rara National Park but local people can use natural resources, including grazing, within the buffer zone (Durga Poudel, Chief Warden, pers. comm.). Nevertheless, we recorded evidence of livestock in our survey plots within the Park and observed buffalo, cattle, horses, goats and sheep in the Park. Many livestock graze in the Park during spring–autumn (Bir Bahadur Buda, Park Staff, pers. comm.). Park officials gather livestock in the Park and confine them to punish their owners but often the owners do not claim them. The livestock are later released outside the Park but often return shortly afterwards.
Livestock grazing probably reduces bamboo abundance and availability (Williams, Reference Williams2003). Red pandas may forage in areas livestock cannot access (e.g. on steeper slopes; Wei et al., Reference Wei, Feng, Wang and Hu2000) but we did not have the data to investigate this. Jotikapukkana et al. (Reference Jotikapukkana, Berg and Pattanavibool2010) also demonstrated, in Thailand, a negative association between wildlife presence and livestock activity.
Threats associated with livestock grazing, including herders and their livestock-guarding dogs, which chase and kill red pandas, may have a greater effect on red pandas than grazing (Yonzon, Reference Yonzon1989). At least six red pandas were killed by hunters and herders near Rara National Park during March–July 2012 (The Kathmandu Post, 2012). Furthermore, dogs may kill red pandas, introduce disease, and reduce the presence of the panda in otherwise suitable habitats (Williams, Reference Williams2003; Lenth et al., Reference Lenth, Knight and Brennan2008). We observed people collecting bamboo illegally in the Park, for fodder and building materials, as occurs in other protected areas of Nepal (Yonzon & Hunter, Reference Yonzon and Hunter1991; Sharma & Belant, Reference Sharma and Belant2010) and Bhutan (Dorji et al., Reference Dorji, Rajaratnam and Vernes2012), and such activities may reduce bamboo availability for red pandas.
Although bamboo can comprise > 90% of the red panda's diet (Wei et al., Reference Wei, Feng, Wang and Hu1999) bamboo alone did not influence red panda occurrence (Table 2). Although bamboo is important for the red panda, this may not have been detected at the fine spatial resolution we evaluated. Elevation did not influence red panda occurrence, probably because our entire study area was within the typical elevational range reported for this species (Choudhury, Reference Choudhury2001). The climate on north-facing slopes at these elevations provides more suitable conditions for bamboo than do south-facing slopes (Numata, Reference Numata1979; Yonzon et al., Reference Yonzon, Jones and Fox1991). The absence of bamboo and red pandas on south-facing slopes suggests that bamboo was more important than elevation for determining the species’ occurrence. Although red pandas drink water soon after eating (Yonzon, Reference Yonzon1989), distance to water did not influence red panda presence, probably because most of the survey plots were ⩽ 100 m from water.
Based on our findings we make three recommendations: (1) enforcement of existing regulations regarding livestock grazing in Rara National Park (if livestock use cannot be restricted we suggest that grazing zones, where livestock can be monitored by herders, be restricted to south-facing slopes where red pandas do not occur); (2) that training workshops be held for herders, to encourage them to develop husbandry practices that include individual or communal livestock corralling, and establishment of areas with supplemental fodder within the buffer zone, to reduce grazing within the Park; (3) dissemination of pamphlets and dialogue with residents of the Village Development Committees and visitors, to inform all users of the Park of the threats of livestock to the red panda, and associated regulations. Reducing the use of Rara National Park by livestock, to enhance the conservation of the red panda, will require increased public awareness, community support, and enforcement of regulations.
Acknowledgements
The Rufford Small Grant Foundation, Norwegian University of Life Sciences, International Fur Trade Federation, and Mississippi State University provided funding. The Department of National Parks and Wildlife Conservation, Nepal, permitted this research. We thank Dr Mahendra Maharjan, Ravi Sharma, Bir Bahadur Buda, Raju Rimal and Rara National Park staff for their support. The comments from two anonymous reviewers helped to improve the article; we appreciate their time and effort.
Biographical sketches
Hari P. Sharma studies the distribution and conservation of red pandas in Nepal and is interested in carnivore ecology. Jerrold L. Belant studies carnivore ecology, human–wildlife interactions, and international conservation. Jon E. Swenson’s interests include the effects of human activities on wildlife. He is now concentrating on brown bear ecology and conservation in Europe.







