INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) is one of the dominant foodborne zoonotic pathogens worldwide [Reference Beutin1, Reference Hyochin and Arun2]. The pathogenic potential of STEC is attributable to shiga toxins 1 and 2, encoded by stx1 and stx2 genes [Reference Paton and Paton3]. In STEC the significance of the O157 serogroup in causing haemorrhagic colitis and haemolytic uraemic syndrome (HUS) in humans is well established [Reference Griffin and Tauxe4, Reference Mead and Griffin5]. Livestock, especially cattle, are natural reservoirs of STEC where they colonize without causing clinical signs [Reference Hancock6]. Reports, mostly on STEC O157, are available on cattle reared in organized farms [Reference Mechie, Chapman and Siddons7, Reference Schouten8]. There are also a few reports on STEC from milk and pooled faecal samples from household cattle in Kenya and Brazil [Reference Kang'ethe9, Reference Lira, Macedo and Marin10]. However, to the best of our knowledge, the carriage intensity of STEC in cattle on smallholdings, based on direct faecal samples from the rectum, and its impact on public health have not been studied before in any developing countries, including Bangladesh.
Selective media have been developed for identifying STEC O157 serogroup [Reference Nataro and Kaper11]. The most common is cefixime and potassium tellurite-supplemented sorbitol MacConkey agar (CT-SMAC) [Reference Nataro and Kaper11–Reference Muller15]. The E. coli O157 serogroup do not ferment sorbitol, and consequently grow as colourless colonies following 24 h incubation, unlike typical E. coli [Reference Manafi and Kremsmaier16]. However, some sorbitol non-fermenting (SN-F) non-O157 strains have been reported [Reference Ojeda17]. Intimin, an outer membrane protein, encoded by the eae gene, is a virulence-associated factor that causes attaching and effacing lesions in the intestinal mucosa [Reference Kaper18]. Another factor contributing to STEC virulence is enterohaemolysin, a pore-forming cytolysin encoded by a plasmid-borne gene designateded hlyA [Reference Schmidt, Beutin and Karch19]. The presence of both stx1 and stx2 genes and the eae gene is a potentially highly virulent combination [Reference Franz20]. Molecular characterization of STEC to identify their diversity by the distribution of the two shiga toxin-producing genes – stx1 and stx2 – in association with other key virulent genes, such as eae and hlyA circulating in cattle on smallholdings are important in order to predict the potential threats of STEC to public health, and to suggest approaches to mitigate the threats at source. Based on the distributions of key virulent genes, mentioned above, we describe herein the molecular characteristics of STEC from cattle on smallholdings in Bangladesh by investigating the SN-F bacteria growing on CT-SMAC agar from faecal samples collected directly from the rectum.
MATERIALS AND METHODS
Study population, sampling and samples
About 50% of village smallholdings in Bangladesh have bovine animals [21], although their numbers vary. They live close to human dwellings, exposing human drinking water and food to faecal contamination. We investigated SN-F STEC in cattle on such rural smallholdings in Bangladesh. One of the six divisions of Bangladesh, Chittagong, in the South-East, was selected for sampling. Eleven districts were used as the sampling frame from which three were selected by lottery: Chittagong, Feni and Noakhali. In hierarchical order these three districts were the first stratum of sampling; also sampled within these districts were eight sub-districts (upazilas) (second stratum), within these sub-districts 21 villages (primary sampling units), within these villages 371 cattle smallholdings, and finally within these smallholdings 518 individual animals. The proportion of cattle harbouring SN-F STEC was assessed at the overall population level, not by distinct clusters based on the differences in herd size because such differences are small as most cattle smallholdings in Bangladesh have only 1–3 animals.
From each smallholding at least one animal was sampled. For each animal a sterile swab was inserted into the anus and the rectal wall was swabbed. The swab was placed in a 5 ml tube containing buffered peptone water (Oxoid, UK), and sent to the Microbiology Laboratory, Chittagong Veterinary and Animal Sciences University (ML-CVASU), Bangladesh. Additional demographic and epidemiological information (age, sex, breed, health status and history of use of antibiotics) from each smallholding was collected.
Bacteriological investigations
For initial screening of SN-F STEC, CT-SMAC agar (Oxoid) was used, which is a selective medium for STEC O157 serogroup, indicated by the growth of colourless colonies [Reference Thrusfield22–Reference Wells24]. Each sample was streaked onto a CT-SMAC agar plate, incubated at 37°C for 24 h. At least five colourless colonies were transferred to a 10 ml test tube containing 5 ml of tryptic soy broth (TSB, Oxoid), and incubated at 37°C for 6 h with continuous shaking. The growth in TSB was inoculated onto CT-SMAC agar again and when only homogeneous colourless colonies (Fig. 1) were observed on it, multiple cross-sectional colonies were verified for E. coli based on standard bacteriological procedures. Confirmed E. coli isolates were preserved at −80°C in LB broth with 15% glycerin.
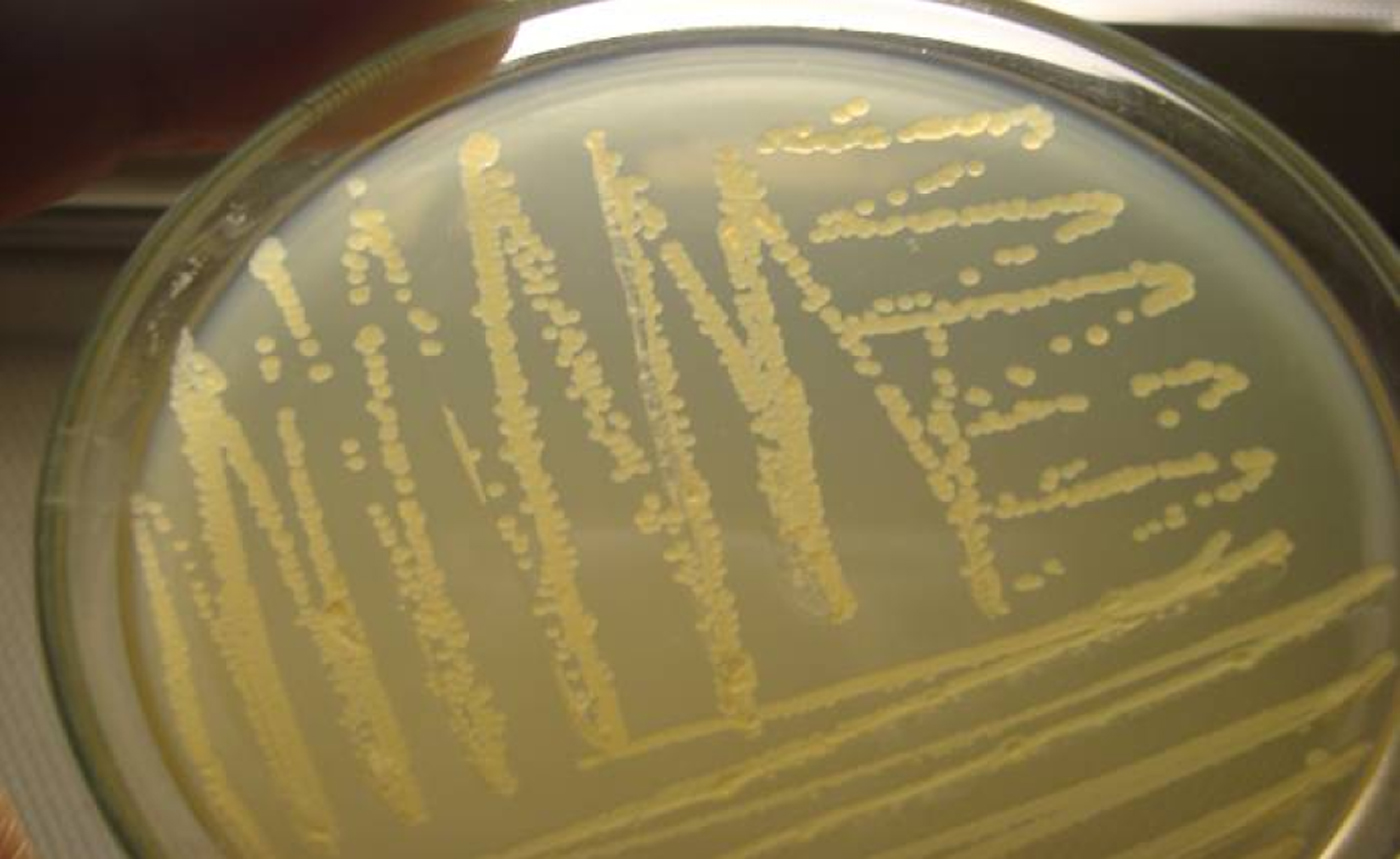
Fig. 1 [colour online]. Secondary homogeneous growth of sorbitol non-fermenting (SN-F) bacterial colonies on cefixime-potassium tellurite sorbitol MacConkey (CT-SMAC) agar, plated from the culture in tryptic soy broth obtained from multiple (⩾5) SN-F colonies inoculated into it from the primary SN-F bacterial colonies developed along with sorbitol-fermenting colonies and others on CT-SMAC agar from a rectal swab collected from a bovine animal on a smallholding in Bangladesh
Verification of O157 serogroup and detection of shiga toxin-producing and other virulence genes
Each SN-F E. coli isolate was screened by polymerase chain reaction (PCR) for the rfb gene to verify whether it belonged to the O157 serogroup. The presence of two shiga toxin-producing genes, stx1 and stx2 and two other virulence genes, eae and hlyA was also investigated by PCR. The sequences of five sets of primers used for the detection of these five genes and their amplicon sizes are shown in Table 1.
Table 1. Sequences of the primer sets used to detect five genes: rfb, stx1, stx2, eae and hlyA in sorbitol non-fermenting Escherichia coli from cattle on smallholdings in Bangladesh

For PCR, DNA from the selected SN-F colonies was extracted by boiling, as described by Sánchez et al. [Reference Sánchez29]. Briefly, a loopful of bacterial growth was suspended in 0·5 ml deionized water, boiled for 5 min to release the DNA, and centrifuged at 15 000 g for 2 min, next 100 μl of the supernatant was collected in another tube to be used as DNA template. Uniplex PCR was performed separately for the detection of each of the five genes. Amplification of a gene was performed in 50 μl of reaction mixture containing 5 μl of 20 mm magnesium chloride, 1 μl of 40 μ m dNTPs, 1 μl of 20 pmol of each primer, 0·2 μl of Dream Taq DNA polymerase (0·4 U/μl) (Thermo Scientific, Fermentas International Inc., USA), 1 μl of DNA template and 40·8 μl of molecular grade water. The conditions for PCR consisted of an initial denaturation step at 95°C for 3 min, followed by 30 cycles of denaturation at 95°C for 20 s, annealing at 58°C for 40 s, and extension at 72°C for 1 min, performed on a Thermo-cycler (2720 Thermal cycler, Applied Biosystems, USA). The amplicon from a PCR for a particular gene was analysed by electrophoresis on a 1·5% agarose gel stained with ethidium bromide (0·5 μg/ml) (Sigma-Aldrich, USA), and visualized by a UV trans-illuminator (BDA digital, Biometra GmbH, Germany). The amplicon size of a gene was determined by including a 1 kb size DNA marker (O'GeneRuler 1 kb plus; Thermo Scientific Fermentas, USA) in each gel. DNA from a previously isolated strain harbouring the particular gene, preserved at ML-CVASU was used as a positive control. A blank reagent having all components except the template DNA was used as a negative control.
Testing for clonal relationship of the isolates without virulence genes
Seven of the eight strains that had none of the virulence genes (stx1, stx2, eae, hlyA) investigated in this study were sent to the Department of Veterinary Disease Biology, Copenhagen University, Denmark, and investigated for clonal relationship by pulsed-field gel electrophoresis (PFGE) following the standardized CDC PulseNet protocol [30]. Briefly, an overnight culture of bacteria grown on brain heart infusion (BHI) broth (Oxoid) was used. Genomic DNA was prepared using 1% agarose (SeaKem® gold agarose, Lonza, USA) and embedded DNA was digested using 60 U of restriction enzyme XbaI (New England BioLabs Inc., USA) for 14 h at 37°C. The DNA fragments were isolated by electrophoresis in 0·5x TBE buffer using the CHEF DR III (Bio-Rad Laboratories, USA) system at 14°C with initial switch time 2·2 s, final switch time 54·2 s, current 6 V/cm, included angle 120° and run time 19 h. The gel was stained with 1% ethidium bromide (Sigma-Aldrich) solution for 30 min and destained in deionized water three times at 20-min intervals. Using UV transillumination, a gel image was captured by the GelDoc EQ system with Quantity One® v. 4.2.1 software (Bio-Rad Laboratories) and images were saved in TIFF format in a computer. The analysis of the fingerprints was performed using GelCompar® II v. 4.6 software (Applied Maths, Belgium). Dice coefficient with a band position tolerance of 1% and 0·5% optimization level were used to determine similarity between fingerprints. The unweighted pair-group method with arithmetic averages (UPGMA) was applied to produce the dendrogram. The DNA restriction patterns of the isolates were interpreted as described by Tenover et al. [Reference Tenover31].
Statistical analysis
All data were entered into a spreadsheet program (Excel 2003, Microsoft Corporation, USA) and transferred to Stata v. 11.0 (Stata Corp., USA) for analysis. The proportion of cattle positive with a particular phenotype/genotype of E. coli was calculated based on the number of animals found positive with this phenotype/genotype as the numerator and the total number of animals sampled as the denominator. The 95% confidence interval (CI) of a proportion estimate was calculated using the syntax ‘cii #exposure #events, level (95) wilson’.
RESULTS
Overview of the study population
In a hierarchical order the sampling levels are shown in Table 2 with the villages as the primary sampling units. The numbers of bovine animals sampled on the selected smallholdings in the villages and the corresponding number of sampled animals positive for SN-F STEC are also presented. The number of smallholdings sampled was 371 and the total number of cattle owned by the selected households on the days of sampling was 789. The median number of cattle held was two (minimum one, maximum six). Of the 518 cattle sampled, 448 were local/indigenous (non-descriptive) type, and 70 cross-bred from indigenous dams.
Table 2. Sampling levels – hierarchically from district to village, numbers of cattle sampled on the selected smallholdings and the corresponding number of sampled animals positive for shiga-toxin producing sorbitol non-fermenting Escherichia coli (SN-F STEC) in the study

District 1, Chittagong; district 2, Feni; district 3, Noakhali [there are 64 districts and 482 sub-districts (upazilas) in Bangladesh].
Proportion of cattle positive with SN-F STEC
A total of 127 swabs yielded multiple SN-F (colourless) colonies on CT-SMAC agar plates, interspersed with others including sorbitol-fermenting (SF) (pink) ones. When cultures from at least five colourless homogeneous colonies in TSB were plated onto CT-SMAC agar, 57 yielded pure SN-F (colourless) colonies, and 44 were identified as E. coli, giving a proportion of 8·5% (95% CI 6·4–11·2). Only two isolates were positive for the rfb gene, a very low proportion (0·4%, 95% CI 0·1–1·4) of the O157 serogroup; however, five and 26 isolates carried the stx1 and stx2 genes, respectively (Table 3); hence, the proportion of cattle carrying SN-F shiga toxin 1- and shiga toxin 2-producing E. coli was 0·9% (95% CI 0·4–2·2) and 5% (95% CI 3·4–7·3), respectively. Of the STEC isolates three had both stx1 and stx2 genes; therefore, a total of 28 samples (5·4%, 95% CI 3·8–7·7) were positive for STEC. Of the 28 STEC, irrespective of whether they harboured stx1 or stx2 or both genes, 13 were also positive for the hlyA gene, and 18 carried the eae gene. On the other hand, eight SN-F non-STEC isolates were positive for the hlyA and/or the eae genes. PCR results illustrating amplicons of some SN-F E. coli isolates harbouring stx1, stx2, hlyA, eae and rfb genes are shown in Figure 2(a–e).

Fig. 2. Results of polymerase chain reaction assays displaying amplicons of the shiga toxin 1-producing gene (stx1) in five (a), shiga toxin 2-producing gene (stx2) in seven (b), enterohaemolysin-producing gene (hlyA) in eight (c), intimin-producing gene (eae) in eight (d) and a somatic antigen-producing gene indicating presence of any member belonging to O157 serogroup (rfb) in two (e) of the sorbitol non-fermenting Escherichia coli strains (indicated by an asterisk in Table 3) from cattle on smallholdings in Bangladesh. Lane M, 1 kb plus DNA marker; lane P, positive control isolates having the specific gene of the concerned panel; lane N, negative control.
Table 3. Distribution of shiga toxin producing genes, stx1 and stx2, and two other virulent genes, eae and hlyA, in the 44 sorbitol non-fermenting strains of Escherichia coli resulting from a survey encompassing 518 cattle on smallholdings in Bangladesh using cefixime potassium tellurite sorbitol MacConkey agar as the initial screening medium

A, Adult; C, calf; +, presence of the gene; −, absence of the gene; * isolates: amplicons of the respective investigated genes which are displayed in Figure 2.
District 1, Chittagong; district 2, Feni; district 3, Noakhali.
According to geography 16, two and 10 animals were found positive for STEC in districts 1 (Chittagong), 2 (Feni) and 3 (Noakhali), respectively. In these animals, the male:female ratio was 15:13, with 21 adults and seven calves.
Clonal relationship of the isolates lacking any shiga toxin, hlyA and eae genes
Among the seven isolates tested, six different pulsotypes were identified based on the variations in >9 bands (i.e. >3 genes) (Fig. 3).

Fig. 3. Dendrogram showing the cluster analysis on the basis of XbaI pulsed-field gel electrophoresis (PFGE) of the seven sorbitol non-fermenting Escherichia coli isolates from cattle on smallholdings in Bangladesh lacking the shiga toxin genes, stx1 and stx2 and two other virulence genes eae and hlyA. Dice coefficient was used to perform similarity analysis, and clustering was performed by using The unweighted pair-group method with arithmetic means with 1% band position tolerance and 0·5% optimization parameter.
DISCUSSION
Approximately 5·4% of cattle on smallholdings in the study area carry SN-F STEC. Of the 28 SN-F STEC isolates 13 (46·4%) and 18 (64·3%) had the virulence gene hlyA and eae, respectively. The proportion harbouring STEC O157 appears to be negligible, only 0·4%. Based on the distributions of two shiga toxin-producing genes stx1 and stx2, and the presence of the two virulence genes eae and hlyA, the SN-F STEC isolates obtained appear highly diverse. The SN-F E. coli isolates lacking any of the four virulence genes – stx1, stx2, eae and hlyA – were genetically diverse. To the authors' knowledge, this might be the first report on SN-F STEC from cattle on smallholdings from Bangladesh or any part of the developing world, to analyse faecal samples collected directly from the rectum.
The aim of the study was to assess the proportion of cattle harbouring STEC at the overall population level, not its carriage rate at any cluster or subpopulation level, on the basis of herd size or different exposure variables such as age, breed, sex, health status and use of antibiotics. A higher sample size from a specific sub-population is required to estimate the prevalence of STEC in that population.
By the use of CT-SMAC we sought to isolate all bacteria belonging to the STEC O157 serogroup (a widely reported serogroup that can cause colitis, HUS and sometimes death in humans) [Reference Griffin and Tauxe4, Reference Mead and Griffin5] and other SN-F types. This study demonstrates that based on seeing colourless colonies on CT-SMAC, STEC O157 cannot be reliably differentiated from other organisms; therefore other tests must be applied. We used PCR to detect the rfb gene, responsible for the production of a common somatic antigen of the O157 serogroup, and only two of the 44 SN-F isolates obtained from the survey belonged to the STEC O157 serogroup. However, both the isolates harboured only one shiga toxin-producing gene, i.e. stx2.
To increase the probability of isolating STEC O157 or any members of STEC belonging to other serogroups, we examined multiple (⩾5) SN-F colonies that were mixed with other colony types on CT-SMAC. However, SN-F E. coli was identified from only 44 of the 127 samples. Except for two isolates harbouring the rfb gene, O-serotypes of the other isolates were not determined because of resource constraints. Neither were the identities of SN-F bacterial isolates other than E. coli investigated, as this was considered beyond the scope of the study. However, among others Burkholderia, Pseudomonas, Vibrio and Aeromonas have been reported to produce SN-F colonies on CT-SMAC [Reference Muller and Ehlers32]. We did not investigate any of the 13 SN-F strains found to be repeatedly S-NF but non-E. coli for the presence of hlyA and/or eae genes. Despite the above-mentioned limitations of this study, an important finding of this investigation is that, on smallholdings in Bangladesh, the proportion of cattle harbouring SN-F non-O157 STEC might be much higher than the proportion positive with STEC belonging to serogroup O157.
Compared to shiga toxin 1-producing SN-F STEC isolates the proportion of shiga toxin 2-producing isolates was ~4 times higher in the study population (Table 3), in agreement with Tahamtan et al. [Reference Tahamtan, Hayati and Namavari33] and El-Jakee et al. [Reference El-Jakee34] who reported prevalence in cattle of 10% vs. 53% and 39% vs. 77%, respectively. The prevalence of the stx2 gene in STEC in recently slaughtered cattle sampled at slaughterhouses in Bangladesh was reported to be >93%, although its presence, compared to the stx1 gene in non-O157 STEC, was found to be lower [Reference Islam35]. SF STEC strains belonging to serotype O157:H– (non-motile) have emerged as important causes of human disease [Reference Pollock36–Reference Orth38] with some evidence that they are more frequently associated with HUS than are SN-F strains [Reference Orth38, Reference Alpers39]. Because the aim of the study was to investigate shiga toxin-producing SN-F STEC, the rate at which the study population harboured SF STEC was not determined.
Because of unrepresentative sample sizes at the sub-population level the isolation trends of STEC in adult animals compared to the calves and in males compared to females could not be predicted from this study. However, one male and two female animals belonging to three different smallholdings were found to be reservoirs for SN-F STEC carrying the stx1, stx2 and eae genes, a potentially highly virulent combination [Reference Franz20].
Most STEC isolates resulting from this study were genetically diverse because of having different combinations of hlyA, eae and shiga toxin-producing genes (Table 2), as previously mentioned. Therefore, we did not apply PFGE to discriminate them further. However, Islam et al. [Reference Islam35] reported at least 37 different PFGE patterns in the STEC strains isolated from cattle slaughtered in Bangladesh, indicating heterogeneous clonal diversity. Because eight of the SN-F E. coli isolates lacking any virulence genes could not be discriminated by PCR we applied PFGE to verify whether or not any specific clone of such SN-F non-STEC was circulating in the study population, although the potential of SN-F non-STEC in causing human infections has probably not been documented. The PFGE results reveal that the investigated isolates were genetically diverse (Fig. 3).
In conclusion, ~8% and ~6% of cattle on smallholdings are reservoirs of SN-F E. coli and SN-F STEC, respectively. Most SN-F STEC are non-O157 and only ~0·4% might be STEC O157. Among SN-F STEC, 10·7% should be considered highly virulent to humans because of the stx1, stx2 and eae genes, indicating a threat from cattle on smallholdings to the people sharing the same homestead. The proportion of cattle positive with SF STEC remains unknown. More studies are needed to assess the prevalence of SF STEC and to identify the sources and risk factors associated with SN-F or SF STEC from cattle on smallholdings in human infection.
ACKNOWLEDGEMENTS
The authors thank W. R. Ward for examining the manuscript. The study was financed by RFLDC, Noakhali though the Poultry Research and Training Centre, CVASU, Bangladesh. Thanks are extended to Dr Himel Barua for providing valuable technical support.
DECLARATION OF INTEREST
None.











