n-3 and n-6 long-chain PUFA (LC-PUFA) including DHA and arachidonic acid (AA) serve as indispensable structural components of cellular membranes and are important for normal retinal and neuronal development(Reference Salem, Litman and Kim1, Reference Innis2). Infants have a limited conversion of LC-PUFA from its precursor 18-carbon fatty acids (FA), with high dependence on LC-PUFA provided by antenatal placental transfer and later from breast milk or infant formula(Reference Innis3). As maternal LC-PUFA intake is the major determinant of LC-PUFA transfer to the offspring(Reference Hornstra4), there has been growing concern that the low intakes of n-3 LC-PUFA intake in Western nations, particularly DHA, may place infants at risk of deficiency during critical early period of neurological development(Reference Simopoulos5).
The functional effects of n-3 LC-PUFA upon outcomes such as visual acuity and other neurodevelopmental markers have been explored in many randomised controlled trials of LC-PUFA supplementation in term infants(Reference Drover, Hoffman and Castaneda6–Reference Beyerlein, Hadders-Algra and Kennedy28). Such studies have typically evaluated the effects of LC-PUFA-supplemented formula, which historically contained no preformed LC-PUFA. In breast-feeding infants, there have been trials in which lactating mothers were supplemented to increase LC-PUFA levels derived through breast milk(Reference Helland, Smith and Saarem29–Reference Jensen, Voigt and Prager32). Both methods, however, have provided conflicting evidence on the question of whether postnatal LC-PUFA supplementation is beneficial to neurodevelopment in term infants(Reference Simmer and Patole33, Reference Hoffman, Boettcher and Diersen-Schade34).
There are a number of proposed reasons for the lack of definitive findings in previous research trials. First, previous dosages may have been insufficient to determine differences between treatment groups. Most studies in healthy term infants have raised DHA intakes to approximately that of breast-fed infants in Western nations, between 0·2 and 0·3 % FA in breast milk. In contrast, nations such as Japan report breast milk levels of up to 1 %, probably due to high fish intake(Reference Brenna, Varamini and Jensen35). Currently, there have been no randomised controlled trials to evaluate the effects of direct supplementation with high-dose LC-PUFA (>1 %) in term infants.
Second, there has been a lack of trials that have investigated the effects of directly supplementing infants with n-3 LC-PUFA independently from the feeding method. Agostoni et al. (Reference Agostoni, Zuccotti and Radaelli20) in a recent study provided DHA in liquid form during the first year of life, but provided only 20 mg, chosen as it would be the minimum amount supplied within human milk. Consequently, there have been no trials that have investigated the effects of directly supplementing infants with n-3 LC-PUFA at higher doses. Direct supplementation could be advantageous, as it is applicable to the entire infant population, regardless of the feeding practice.
Lastly, a frequently proposed reason for inconsistencies in previous findings is the use of scientifically inadequate or insensitive methods for developmental assessment. A large percentage of controlled trials include global tests of development, which are not designed to detect subtle changes in brain function(Reference Carlson36). The assessment of language proficiency involving its interdependent aspects of comprehension, gesture, spoken language and appropriate social behaviour, all of which are dependent on integrated neural networks and rapid speed of processing, could be a more suitable domain in which to evaluate nutritional effects on infant development.
To our knowledge, this is the first double-blind, randomised, controlled study to evaluate the effects of fish oil (FO) supplementation for the first 6 months in healthy term infants on neurodevelopment at 18 months, and language development at 12 and 18 months of age. Secondary objectives were to evaluate the effect of FO supplementation on erythrocyte and plasma phospholipid FA at 6 months of age, and associations between FA levels and neurodevelopmental outcomes.
Subjects and methods
The study design and methodology have been described elsewhere(Reference Meldrum, D'Vaz and Dunstan37). A brief summary is provided below.
Subjects
The study population included the infants of 420 women in Perth, Western Australia who were recruited for a double-blind, randomised, controlled study conducted at Princess Margaret Hospital for Children, aimed at assessing the effects of infant FO supplementation on both infant allergy and neurodevelopment. Women were recruited during the third trimester of pregnancy between 1 June 2005 and 1 October 2008 from private and public metropolitan antenatal clinics. Allergic pregnant women were recruited as their infants are at a higher risk of developing allergic disease(Reference Litonjua, Carey and Burge38). Maternal atopy was defined by at least one positive skin prick test to at least one of a defined panel of allergens. Exclusion criteria included maternal smoking, a pre-existing medical condition or high-risk pregnancy, more than three fish meals consumed per week or FO intake during pregnancy in excess of 1000 mg/d, preterm delivery and infants with significant congenital abnormalities or medical conditions.
Study design
Infants were randomised to receive either a high-dose DHA-enriched ethyl ester FO supplement, aimed at delivering 250–280 mg DHA/d (n 218) or an image-matched placebo containing olive oil (n 202). The main supplier for the study was Ocean Nutrition, Canada Limited. Capsules were purchased in one batch in 2005, and the composition was regularly tested by an independent laboratory during the trial. The FO capsules contained 650 mg FO comprising 280 mg DHA and 110 mg EPA. The placebo capsules contained 650 mg olive oil (66·6 % n-9 oleic acid). The FA composition of the capsules remained unchanged over the course of the study and peroxide and acid levels remained compliant with Australian standards. Because the trial took longer than anticipated, and because Ocean Nutrition discontinued the original products, the final forty-three children received capsules donated from Numega. These TAG FO capsules contained a similar amount of DHA (260 mg DHA and 60 mg EPA) and the placebo capsules were also olive oil. This brand substitution was endorsed and supervised by the Ethics Committee at Princess Margaret Hospital. Both capsules were image and scent matched. There was no significant difference between the erythrocyte DHA (P =0·732) or plasma phospholipid DHA (P =0·160) levels between the two groups of participants receiving FO capsules from different suppliers, so all children were included in the final analysis.
Supplementation commenced at birth and ceased at 6 months of age. Although the primary outcomes were analysed on an ‘intention-to-treat’ basis, we also examined and allowed for differences in capsule consumption between the groups (see below). Capsule consumption/adherence was based on capsule diaries and a count of the returned capsules, in addition to the infant FA analysis (see below).
Randomisation and the allocation of capsules were completed by external staff (Princess Margaret Hospital Pharmacy) via computer software using an unpredictable allocation sequence, stratified according to maternal atopy, paternal atopic history and parity. Mothers and study personnel were unaware of the group allocation. Intervention and control capsules were image matched, and packaged in identical containers labelled with the participant's name and birth date.
The dose was chosen as a high dosage in comparison with previous trials studying the effects of n-3 LC-PUFA supplementation in infancy, under the assumption that this would optimise the likelihood of detecting biologically or clinically relevant effects of supplementation. It was recommended that capsules be given to babies in the morning immediately before breast-feeding (capsule pierced and squirted into the infant's mouth), or in the formula during their first daily feed.
Results of the allergic and immunological outcomes will be published elsewhere. Information regarding weight, height, head circumference, relevant medical diagnosis and mode of feeding (including brand of infant formula) was collected at each clinical visit. Demographic and obstetric data were collected at birth and participants were seen at 3, 6, 12 and 18 months.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Princess Margaret Hospital Research and Ethics Committee. Written informed consent was obtained from all participants.
Fatty acid analysis
Where possible, two blood samples were collected (1) from cord blood at birth and (2) from infants at age 6 months, following the supplementation period. Phospholipid FA composition was measured in both erythrocyte cell membranes and plasma to provide an indication of FA status over the preceding 6 months(Reference Fekete, Marosvolgyi and Jakobik39). Fatty acid compositional analysis was completed as described previously(Reference Mori, Burke and Puddey40). Briefly, peripheral and cord blood erythrocytes were collected into heparinised Roswell Park Memorial Institute (RPMI) medium and cells separated using density centrifugation. Erythrocytes were isolated, washed and stored at − 20°C in methanol–chloroform (2:1) and lipids were extracted by TLC. Plasma (0·5 ml) was extracted with chloroform–methanol (2:1, by volume, 5 ml) and the phospholipid fraction was obtained from total lipid extracts by TLC. Fatty acid methyl esters were prepared by treating phospholipid extracts with 4 % H2SO4 (by volume) in methanol at 100°C for 10 min and analysed by GLC on an Agilent 7890 Series Gas Chromatograph System equipped with an Agilent 7683 Autosampler and an Agilent 7683B Autoinjector Module (Agilent Technologies Australia Private Limited). Chromatography was performed on a Supelco SP-2560 column (100 m × 0·25 mm × 0·20 μm; Sigma-Aldrich Private Limited) using hydrogen as the carrier gas at a split ratio of 30:1. The temperature programme was 200 °C for 8·5 min, then 8 °C/min to 240 °C and held for 4·5 min. Peaks were identified by comparing them with a known standard mixture. Individual FA were calculated as a relative percentage with the evaluated FA set at 100 %.
Breast milk fatty acid analysis
Maternal breast milk samples were collected at 3 and 6 months of age either by manual expression or by breast pump. They were frozen immediately and stored at − 80°C until analysis. Fatty acid analysis was performed on the thawed whole milk samples as described previously(Reference Dunstan, Roper and Mitoulas41). Briefly, lipids were extracted and transmethylated using 1 % H2SO4 in methanol. Fatty acid methyl esters were separated using a gas chromatograph with flame ionisation detection using a modification of the previously published method(Reference Makrides, Neumann and Simmer42). Fatty acids were identified based on retention time to authentic lipid standards and expressed as mean percentages and standard deviations of the total FA measured.
12-month assessment
Macarthur–Bates Communicative Development Inventories (Words and Gestures) (MCDI)(Reference Fenson, Dale and Reznik43) (see below) were posted to the parents of the infants to complete before the 12-month clinical visit for allergy.
18-month assessment
Participants were invited to attend a neurodevelopmental appointment at 18 months of age. The assessments were completed by two assessors (rater 1: n 50, rater 2: n 237) trained in the administration of the tests in a quiet room. The following assessments were completed:
(1) Bayley Scales of Infant and Toddler Development (3rd edition; BSID-III)(Reference Bayley44) is an internationally recognised tool to assess the development in very young children (1–42 months). It consists of three scales administered with child interaction including cognitive, motor (consisting of fine and gross motor skill) and language (consisting of expressive and receptive language); and two scales conducted with parent questionnaires including social–emotional and adaptive behaviour. Raw scores of successfully completed items are converted to scale scores (10 (sd 3)) and to composite scores (100 (sd 15)).
(2) Achenbach Child Behavior Checklist (CBCL)(Reference Achenbach and Rescola45) was used to assess mental health and behavioural development. It measures parental perceptions of child competencies and behaviours in children aged 18 months to 5 years. Scales include affective problems, anxiety problems, attention deficit/hyperactivity problems, oppositional defiant problems and pervasive developmental problems. T-scores are obtained for each of these scales based on the number and extent of the behaviours observed (50 (sd 10)).
(3) Macarthur–Bates Communicative Development Inventory (Words and Gestures) (MCDI)(Reference Fenson, Dale and Reznik43) is a standardised parent reporting system used to assess monolingual children's lexical/vocabulary growth. The infant scale looks at comprehension, word production and aspects of symbolic and communicative gesture. Percentile ranks are computed from age (months) and sex-specific norms.
Statistics
Differences between the groups were determined by independent t test where data were normally distributed, with comparisons being expressed as means and standard deviations. Where possible, non-parametric data were natural log-transformed to achieve a normal distribution as confirmed by the Kolmogorov–Smirnov test and probability plots. If data could not be normalised in this way, non-parametric Mann–Whitney U tests were used. Correlative studies using Pearson's or Spearman's correlations (depending on data normality) were used to assess relationships between neurodevelopmental assessment results and individual FA proportions in cord blood and infant blood samples (in the combined study population). Linear regression analysis was used to determine significant predictors of neurodevelopmental test scores at the 18-month clinical visit, controlling for relevant covariates. All statistical analyses were performed using SPSS software (version 16 for PC). A P value of < 0·05 was considered statistically significant for all FA analyses.
Results
Study participation
The trial design and number of individuals at each stage are shown in Fig. 1. A total of 420 infants were randomised. In total, sixty-six infants withdrew following randomisation; forty-one from the treatment group and twenty-five from the placebo group. An additional number of participants could not be contacted to attend a follow-up visit, despite approximately three attempts via phone or letter. These participants were described as ‘Did not attend visit’ and are documented in Fig. 1 together with reasons for withdrawal. More participants in the treatment group withdrew compared with the placebo group, with FO associated with more withdrawals as a result of the smell. Overall, 287 were assessed for neurodevelopment, 149 from the placebo group and 138 from the FO group.

Fig. 1 Flow chart for study design, participant progress and data collection.
Adherence and blinding
The parents and the investigators remained blinded to the intervention until all the assessments were completed. Before unblinding, the parents were asked to indicate which supplement they believed they had been allocated to. Parents tended to base their assumption on the smell of the capsules, despite the use of peppermint flavouring in both oils; 92·2 % of participants in the FO group correctly guessed their allocation, compared with 56·25 % of the placebo group. As aforementioned, the adherence to the supplementation was based upon a capsule count of the returned capsules and a daily diary kept by the participants. There was a statistically significant difference between the treatment groups for adherence (t (322) = 2·129, P =0·034), with the FO group having significantly lower percentage adherence (55·3 (sd 33·2)) compared with the placebo group (62·6 (sd 28·4)). This was therefore assessed as a confounding factor in the subsequent analysis.
Population characteristics
There were no significant differences in the characteristics of the groups for any of the parameters examined including maternal age, parity, gestational length, neonatal anthropometric measures or incidence of allergic disease (Table 1). The population tended to be predominantly Caucasian, of high income, with high levels of both paternal and maternal education.
Table 1 Population characteristics of the infants in the study population (Percentages, mean values and standard deviations)

LC-PUFA, long chain PUFA; BSID-III, Bayley Scales of Infant and Toddler Development (3rd edition); MCDI, Macarthur–Bates Communicative Development Inventory; CBCL, Child Behavior Checklist.
Due to the reduced number of participants who completed the neurodevelopment assessments relative to the total number of study participants, a comparison of the population characteristics of both groups was completed. For those participants who completed the BSID-III and CBCL, the Apgar score at 5 min was significantly higher (9·17 (sd 0·52)) when compared with those who did not attend (8·99 (sd 1·0); P =0·026), and both the maternal (attended: 33·5 (sd 4·3); did not attend: 31·5 (sd 4·8); P =0·001) and paternal (attended: 35·9 (sd 4·9), did not attend: 34·2 (sd 5·6); P =0·002) ages were similarly higher for those participants who attended. For those who completed the MCDI (either 12 month or 18 month), only maternal age was significantly higher for those who completed the visit (attended: 33·6 (sd 4·3); did not attend: 32·3 (sd 4·6); P =0·004).
While the parental age of the participants who completed the assessment was increased, the income level, the number of siblings, the parental education level, if any other language are spoken at home, and the sex of the participants remained unchanged. Therefore, we can be reasonably confident that the group who completed the assessments is not likely to significantly differ from those who did not attend, and ensure the external validity of the study.
Fatty acid measurements
The erythrocyte FA measured at the end of the 6-month supplementation period showed significantly higher DHA (P =0·03) and EPA (P =0·016) levels and significantly lower AA levels (P =0·003) in the FO group compared with the placebo group (Table 2). The plasma phospholipid FA showed significantly higher DHA (P = 0·001) and EPA levels (P < 0·001), but AA levels remained unaltered (P = 0·376). Such results demonstrate that the supplementation was effective in significantly altering FA of the FO group. The levels of oleic acid in erythrocytes (P = 0·636) were not significantly different between the treatment groups; however, contrary to expectations, oleic acid levels in plasma phospholipids were significantly higher in the placebo group (P = 0·012).
The erythrocyte and plasma phospholipid DHA values of the placebo group (5·78 (sd 1·5); 4·49 (sd 1·3), respectively) were comparable with other Australian breast-fed infants(Reference Makrides, Neumann and Simmer42, Reference Makrides, Neumann and Simmer46). AA levels in plasma phospholipids and erythrocytes within both the placebo and FO groups remained within the range of values comparable with other Australian breast-fed infants(Reference Makrides, Neumann and Simmer42, Reference Makrides, Neumann and Simmer46).
Within the FO-supplemented group, there was a significant correlation between the plasma phospholipid DHA levels at 6 months of age and the adherence to supplementation as measured by the diary/capsule count (r s = 0·426, P =0·001); however, a significant correlation was not observed for EPA or AA. For erythrocyte measurements, the opposite finding was observed, with AA and EPA significantly negatively correlated with adherence (r s = − 0·248, P = 0·05; r s = 0·373, P = 0·003, respectively), and no significant correlation observed for DHA.
We also assessed DHA at birth to confirm that both groups had similar baseline levels before the supplement. There were no significant differences between the groups for the FA measurements taken from cord blood or breast milk (see Table 2). Cord blood DHA and AA levels were comparable with other populations within Western Australia(Reference Dunstan, Mitoulas and Dixon47). The breast milk levels of DHA in the study population (0·34 (sd 15) %) were higher than previously observed levels within Australia of 0·20 % total FA(Reference Brenna, Varamini and Jensen35). This may be due to the high socio-economic status of the participants and awareness of the potential benefits of high FO/fish intake during lactation as a result of study participation. Levels of AA, however, were comparable with previous data(Reference Brenna, Varamini and Jensen35). No EPA could be detected in the breast milk at either time point.
Table 2 Fatty acid measurements (% total fatty acids) taken during the course of the trial for the fish oil and placebo groups (Mean values and standard deviations)
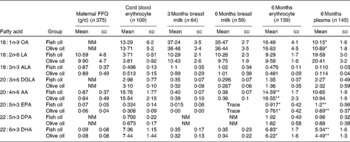
OA, oleic acid; NM, not measured; LA, linoleic acid; ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid; AA, arachidonic acid; DPA, docosapentaenoic acid.
Mean values were significantly different between the fish oil and placebo groups: * P < 0·05, ** P < 0·01.
* Percentage of total fatty acids.
Bayley Scales of Infant and Toddler Development
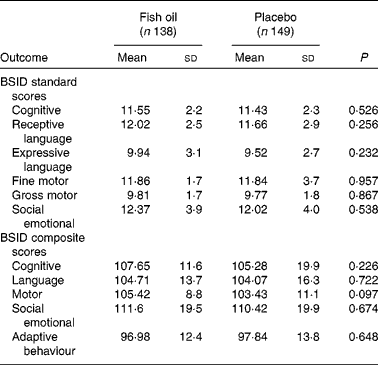
There were no significant differences between the groups for the standard or composite scores in any of the BSID-III subtests. As shown in Table 3, the mean scores for each subtest tended to be higher for the FO group, but this did not reach statistical significance. Of interest to note is the tendency for low scores on the MCDI for this population. One reason for this may be that this is an American assessment, and some of the vocabulary is not appropriate to an Australian population, and this is reflected in the scores being lower when compared with the normative US sample.
Table 3 Bayley Scales of Infant and Toddler Development (3rd edition; BSID-III) outcomes; fish oil compared with placebo at 18 months of age (Mean values and standard deviations)
Child Behavior Checklist
As expected for a typically developing term infant population, the mean scores of both supplementation groups were heavily skewed towards the T-score of 50, the lowest score achievable, and normality could not be obtained statistically. As a result, the data were analysed categorically, with the percentages of children who obtained a T-score>50 comparable between the groups. As shown in Table 4, there was a significant difference between the groups for anxious/depressed behaviours, with more children in the FO group scoring>50 T-score relative to the placebo group (χ2 (1, n 265) = 5·580, P =0·018). This remained significant after adjusting for capsule compliance (β = 0·5, 95 % CI 1·014, 2·838, P =0·044). As shown in Tables 4 and 5, there were no significant differences between the groups in any of the remaining CBCL subscales. There were also no differences in the number of participants in the borderline or clinical range for the CBCL subtests between the study groups (data not shown), either before or after adjusting for capsule compliance.
Table 4 Categorical Child Behavior Checklist outcomes; fish oil compared with placebo at 18 months of age
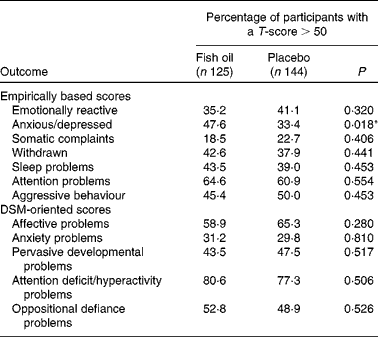
DSM, Diagnostic and Statistical Manual of Mental Disorders.
*P < 0·05.
Table 5 Linear Child Behavior Checklist outcomes; fish oil compared with placebo at 18 months of age (Mean values and standard deviations)

Macarthur–Bates Communicative Development Inventory
Overall, a lower number of participants were able to complete the MCDI, as the use of this questionnaire was not introduced until October 2007, and therefore the first 224 participants did not complete the 12-month questionnaire, and the first 164 participants did not complete the 18-month questionnaire.
As illustrated in Table 6, children in the FO group performed significantly better in language assessments at 12 and 18 months of age with higher percentile ranks of both later developing gestures (P =0·007; P =0·002, respectively) and the total number of gestures (P =0·023; P =0·006, respectively) compared with placebo. These relationships remained significant after adjusting for potential confounding effects of capsule compliance (later developing gestures at 12 months: β = 0·247, 95 % CI 3·433, 24·214, P =0·010; later developing gestures at 18 months: β = 0·224, 95 % CI 3·604, 22·670, P =0·007; total number of gestures at 12 months: β = 0·238, 95 % CI 2·846, 23·642, P =0·013, total number of gestures at 18 months: β = 0·211, 95 % CI 2·685, 21·103, P =0·012). No other significant differences were observed for the remainder of the data collected using the MCDI. While the authors of the MCDI advise that for young infants that small differences in raw scores can produce large shifts in percentile scores(Reference Fenson, Dale and Reznik43), in the present analysis, raw scores were also evaluated statistically, and the same significant differences for the gestural subtests were observed (data not shown). The change between raw scores (δ) at 12 months and 18 months for each subscore of the MCDI was evaluated statistically to evaluate whether the supplementation had any effect upon the rate of language acquisition. No significant effect of group was observed for the δ for any of the MCDI subscores (data not shown).
Table 6 Macarthur–Bates Communicative Development Inventory raw scores and percentile ranks; fish oil compared with placebo at 12 and 18 months of age (Number of participants, mean values and standard deviations)

*P < 0·05, **P < 0·01.
Fatty acid values and neurodevelopmental outcomes
Infant erythrocyte DHA measurements at 6 months of age significantly predicted communication skills in the Adaptive Behaviour Questionnaire of the BSID-III (β = 0·291, 95 % CI 0·115, 0·870, P =0·011) and remained significant after controlling for allergic disease, parity, maternal education, capsule consumption and sex (β = 0·281, 95 % CI 0·043, 0·979, P =0·007). No other significant associations were observed between infant erythrocyte DHA and neurodevelopmental outcomes.
Infant plasma phospholipid measurements at 6 months of age significantly predicted the phrases understood scores in the MCDI questionnaire at 12 months of age (β = 0·468, 95 % CI 3·726, 12·136, P <0·001), and remained a trend, but did not remain statistically significant after controlling for allergic disease, capsule consumption, parity, maternal education and sex (β = 0·310, 95 % CI − 0·080, 10·588, P =0·053).
When we examined the FA at birth, we found no significant associations between cord blood DHA measurements and neurodevelopmental outcomes.
Discussion
The present study is the first randomised controlled trial evaluating the effects of direct high-dose FO supplementation in term infants on neurodevelopment including language acquisition. The findings do not fully support our hypothesis, with no observed effects of FO supplementation on neurodevelopmental skills as assessed by the Bayley Scales. However, in a smaller subset analysis, supplementation resulted in higher gesture scores on the MCDI compared with placebo, both at 12 and 18 months of age. As gesture precedes spoken work acquisition and is associated with later developing vocabulary skills(Reference Goodwyn and Acredolo48), such data study presents a case for future larger multi-centre studies to confirm this effect and address longer-term outcomes of language development. Additionally, the increased score in the anxiety/depression subscale of the CBCL in the present trial does not support a beneficial effect of early FO supplementation on mood.
There have been several previous trials of LC-PUFA supplementation in term infants that have investigated language outcomes, yet results from these trials have not demonstrated consistent benefits to language development(Reference Birch, Garfield and Hoffman14, Reference Auestad, Scott and Janowsky16, Reference Auestad, Halter and Hall17, Reference Lauritzen, Jorgensen and Olsen31, Reference Innis, Gilley and Werker49). Similarly, there is no existing evidence suggesting that enhanced n-3 LC-PUFA status or supplementation with n-3 LC-PUFA is likely to functionally manifest in negative effects upon mental health development. Indeed, in adults, FO supplementation has been noted as protective for mental health(Reference Hallahan and Garland50, Reference Peet and Stokes51). The present findings are also in contrast to the most recent Cochrane review(Reference Simmer and Patole33), which concluded that the majority of well-designed previous trials of LC-PUFA supplementation of infant formula have not shown effects upon neurodevelopment. A number of differences between the present trial and previous studies may have contributed to the observed benefit of postnatal FO supplementation.
First, the method used for the assessment of neurodevelopment could be an important factor. In comparison with global standardised measures of infant development such as the BSID-III, detailed parent-report measures of development such as the MCDI or CBCL represent a cost-effective and highly valid instrument for which to assess a specific facet of cognitive development. Direct assessments evaluate infant skills in a decontextualised environment, which can fail to capture their true functional capacities(Reference Neisworth and Bagnato52). Additionally, the BSID-III is designed to diagnose a delay/disorder in neurodevelopment, which may not be appropriate in determining the potentially subtle effects of a nutritional supplement on normally developing, healthy term infants(Reference Cheatham, Colombo and Carlson53).
Second, the LC-PUFA levels measured at the end of the supplementation period indicated that the increase in DHA status within the treatment group was statistically significant, supporting the hypothesis that supplementation with high-dose FO would significantly increase infant FA status. Furthermore, both infant erythrocyte and plasma DHA status at 6 months of age predicted scores of language/communications skills, lending further support to the group differences between n-3 LC-PUFA and communicative development observed. Finally, the study population is unique in its inclusion of all infants regardless of the feeding practice (i.e. breast or bottle-fed), which increases its wider applicability. Nevertheless, it remains possible that a number of alternative factors may have had an impact on the results observed. Such factors may limit confidence in the group differences observed.
Despite our best efforts to match the capsules, the majority (92·9 %) of participants in the FO group correctly guessed their infant's group allocation. The precise effects of this are unknown, but considering the wide commercialisation of FO capsules as promoting intellectual development, it is feasible that parents within the FO-supplemented group may have altered perceptions of their child's skills. This is unlikely to affect measures such as the BSID-III where the children were assessed by an examiner unaware of the group allocation, but it may have had an impact on parent-report measures such as the MCDI or CBCL.
The discrepant sample sizes between the assessment measures remain a potential for bias within the study. Although with approximately 140 participants per group, the present study did have adequate power to detect differences according to the BSID-III, the number of completed MCDI assessments was lower, at approximately 81 per group. However, this was not due to self-selection bias, as the MCDI was added to the assessment protocol at a later stage of the trial. Still, our sample population was biased to high-income, well-educated, predominantly Caucasian population, which may be expected to have good knowledge of healthy nutrition. This population is also less likely to be ‘deficient’ in DHA, as reflected by the comparatively high levels of DHA in breast milk.
The present trial was part of a large randomised controlled trial designed to assess the role of FO in allergy prevention(Reference Meldrum, D'Vaz and Dunstan37). Infants at high risk of developing allergic disease due to maternal atopy were recruited, as they were at a greater risk of atopy than parents with no family history(Reference Prescott, Macaubas and Holt54). Such a selected population limits the diversity of the study population. Although the exact implications of this population selection cannot be precisely determined, allergic disease has been associated with neurodevelopment in a bidirectional manner(Reference Chida, Hamer and Steptoe55), and this may limit the applicability of the present results for a population not predisposed to allergic disease. Further, despite the fact that randomisation should have ensured that confounding factors were balanced evenly between the groups, an environmental survey or questionnaire such as the HOME inventory at the time of developmental testing would have been beneficial to ascertaining whether any imbalance in the home environment occurred between the randomised groups.
With regard to the erythrocyte LC-PUFA levels, while supplementation successfully increased the DHA status of the treatment group, this increase may be interpreted as modest considering the dose. Gibson et al. (Reference Gibson, Neumann and Makrides30) showed that increasing breast milk levels to approximately 1·13 % DHA resulted in infant erythrocyte phospholipid values of 9·8 % total FA. Considering that the dosage in the present study was approximately 1 % FA, the mean infant erythrocyte DHA value of 6·5 % appears to be lower than expected. One possible reason for this may be capsule adherence, as the FO group reported adherence at a mean of 59 %, thus the dosage is likely to be lower overall than anticipated. A second possible factor is that the majority of the population was already receiving a significant quantity of DHA from breast milk, as DHA levels on average were higher for this cohort than the typical Australian population(Reference Brenna, Varamini and Jensen35). Other considerations include the method of delivery(Reference Liu, Carlson and Rhodes56), which may have resulted in a variable amount of supplement adequately ingested, and the bioavailability of the ethyl ester supplement(Reference Nordoy, Barstad and Connor57, Reference Sala-Vila, Castellote and Campoy58), both of which may have reduced the effectiveness of the supplement in increasing n-3 LC-PUFA levels.
In contrast, the LC-PUFA levels measured in plasma phospholipids indicated that the increase in DHA status within the treatment group was more substantial. However, the statistically significant increase in oleic acid levels in the placebo group does cast doubt as to whether olive oil was an appropriate placebo, which was surprising, taking into account how trivial the amount of oleic acid provided in the supplement compares to the quantity within breast milk or infant formula(59). Plasma phospholipid measurements may reflect short-term intake, therefore compliance may have increased near to the clinical visit in which the sample was taken, as has been observed in previous clinical trials(Reference Cramer, Scheyer and Mattson60).
Several assessment measures were completed within the present trial in order to increase the likelihood of identifying a particular facet of neurodevelopment affected by n-3 LC-PUFA supplementation. However, this increased the type 1 error rate due to the high number of statistical comparisons completed. Although multiple comparisons are inevitable in studies of this nature, the statistical corrections that are often employed to address this (e.g. Bonferroni correction) infer that multiple relationships (even if consistent and significant) detract from each other, and deal with this by adjustments that abolish any findings without extremely significant levels (P values). However, it has been validly argued(Reference Bacchetti61) that where there are consistent, repeated, coherent and biologically plausible patterns, the results ‘reinforce’ rather than detract from each other (even if P values are significant but not very large). We argue that this may be the case with the consistent relationships between n-3 LC-PUFA status and the multiple neurodevelopment measures observed in the present study.
In conclusion, the present findings suggest that FO supplementation during early infancy resulted in higher gestural scores, yet no benefit for overall development or behaviour measured at 18 months in healthy term children. Further, children within the FO group exhibited some evidence of increased anxious/depressed behaviours. However, to confirm such findings, further studies are required with larger sample sizes of a more diverse population. The present study has demonstrated that adherence was reduced using this method of direct supplementation, reinforcing that maternal supplementation during lactation or infant formula may be a more desirable way to achieve higher DHA status, and potentially more conclusive results of the overall effects of high n-3 LC-PUFA supplementation during early infancy may be obtained.
Acknowledgements
This Infant Fish Oil Supplementation Study (IFOS) was funded by the National Health and Medical Research Council (NHMRC) of Australia (grant ID 458502). S. L. P. was supported by a NHMRC Practitioner Fellowship. S. J. M. was supported by a PhD Scholarship from the Women & Infants' Research Foundation. N. D. was supported by a PhD Scholarship from the Asthma Foundation of Australia. We appreciate the contributions of Professor Trevor Mori, Mrs Suzi McCarthy, Dr Meri Tulic, Dr Ylennia Casadio, Dr Elaine Pascoe and Ms Glenys Dixon in addition to the entire Childhood Allergy & Immunology Research Group staff. We also gratefully acknowledge the infants and their families for their participation in this study. The contributions of each author were as follows: S. J. M. was involved in the participant recruitment, data collection, statistical analysis, drafting and editing of the manuscript. S. L. P., J. A. D., K. H. and K. S. conceived and designed the study. N. D. was involved in participant recruitment and data collection. All authors edited the paper content and approved the final manuscript. There are no relevant disclosures pertaining to funding or conflict of interest issues.











