Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Guo, Weihong
Gao, Lei
Chen, Guanlong
Zhang, Yan
He, Haoming
Xie, Tian
Yang, Sanke
Ding, Shimin
and
Yang, Xianjin
2012.
The nonisothermal crystallization kinetics of surface fluorinated polypropylene/polypropylene blend.
Journal of Fluorine Chemistry,
Vol. 144,
Issue. ,
p.
182.
Wellen, Renate M.R.
and
Canedo, Eduardo L.
2014.
On the Kissinger equation and the estimate of activation energies for non-isothermal cold crystallization of PET.
Polymer Testing,
Vol. 40,
Issue. ,
p.
33.
Tohmyoh, Hironori
and
Sakamoto, Yuhei
2014.
Acoustic study of a linear low-density polyethylene film after modification of the crystalline structure by heating.
Review of Scientific Instruments,
Vol. 85,
Issue. 2,
Albo, Jonathan
Wang, Jinhui
and
Tsuru, Toshinori
2014.
Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures.
Journal of Membrane Science,
Vol. 449,
Issue. ,
p.
109.
Wellen, Renate Maria Ramos
2014.
Effect of polystyrene on poly(ethylene terephthalate) crystallization.
Materials Research,
Vol. 17,
Issue. 6,
p.
1620.
Zhao, Jian
Xiao, Changfa
and
Zhang, Zhiying
2014.
Crystallization Kinetics of Polypropylene and Poly (butyl methacrylate-co-hydroxyethyl methacrylate) Blend.
International Journal of Polymer Analysis and Characterization,
Vol. 19,
Issue. 8,
p.
731.
Saeed, Haroon A. M.
Eltahir, Yassir A.
Xia, Yumin
and
Yimin, Wang
2014.
Non-isothermal crystallization kinetics and nucleation activity of hyperbranched polyester (HBPET) in recycled PET.
Polymer Bulletin,
Vol. 71,
Issue. 3,
p.
595.
Wellen, Renate M.R.
and
Canedo, Eduardo L.
2015.
Complex cold crystallisation peaks in PET/PS blends.
Polymer Testing,
Vol. 41,
Issue. ,
p.
26.
Wellen, Renate M.R.
Canedo, Eduardo L.
and
Rabello, Marcelo S.
2015.
Melting and crystallization of poly(3-hydroxybutyrate)/carbon black compounds. Effect of heating and cooling cycles on phase transition.
Journal of Materials Research,
Vol. 30,
Issue. 21,
p.
3211.
Wellen, Renate Maria Ramos
Rabello, Marcelo Silveira
Araujo Júnior, Inaldo Cesar
Fechine, Guilhermino José Macedo
and
Canedo, Eduardo Luis
2015.
Melting and crystallization of poly(3-hydroxybutyrate): effect of heating/cooling rates on phase transformation.
Polímeros,
Vol. 25,
Issue. 3,
p.
296.
Perevedentsev, Aleksandr
Stavrinou, Paul N.
Smith, Paul
and
Bradley, Donal D. C.
2015.
Solution‐crystallization and related phenomena in 9,9‐dialkyl‐fluorene polymers. II. Influence of side‐chain structure.
Journal of Polymer Science Part B: Polymer Physics,
Vol. 53,
Issue. 21,
p.
1492.
Zhou, Lejun
Li, Huan
Wang, Wanlin
and
Chang, Jiang
2018.
Nonisothermal Crystallization Kinetics of Glassy Mold Fluxes.
Metallurgical and Materials Transactions B,
Vol. 49,
Issue. 6,
p.
3019.
dos Santos Silva, Ingridy Dayane
Guimarães Jaques, Nichollas
da Cruz Barbosa Neto, Manoel
Agrawal, Pankaj
Ries, Andreas
Ramos Wellen, Renate Maria
and
Canedo, Eduardo Luis
2018.
Melting and crystallization of PHB/ZnO compounds.
Journal of Thermal Analysis and Calorimetry,
Vol. 132,
Issue. 1,
p.
571.
Wei, Ren
Breite, Daniel
Song, Chen
Gräsing, Daniel
Ploss, Tina
Hille, Patrick
Schwerdtfeger, Ruth
Matysik, Jörg
Schulze, Agnes
and
Zimmermann, Wolfgang
2019.
Biocatalytic Degradation Efficiency of Postconsumer Polyethylene Terephthalate Packaging Determined by Their Polymer Microstructures.
Advanced Science,
Vol. 6,
Issue. 14,
Sousa, Jokdérlea Correa
Arruda, Salim Abdelnor
Lima, Juliana Cisneiros
Wellen, Renate Maria Ramos
Canedo, Eduardo Luis
and
Almeida, Yêda Medeiros Bastos de
2019.
Crystallization kinetics of poly (butylene adipate terephthalate) in biocomposite with coconut fiber.
Matéria (Rio de Janeiro),
Vol. 24,
Issue. 3,
Ferreira Braga, Natália
Morales Zaggo, Henrique
Stieven Montagna, Larissa
and
Roberto Passador, Fabio
2020.
Effect of Carbon Nanotubes (CNT) Functionalization and Maleic Anhydride-Grafted Poly(trimethylene terephthalate) (PTT-g-MA) on the Preparation of Antistatic Packages of PTT/CNT Nanocomposites.
Journal of Composites Science,
Vol. 4,
Issue. 2,
p.
44.
Conrad, Andreas
Hodapp, Annika
Hochstein, Bernhard
Willenbacher, Norbert
and
Jacob, Karl-Heinz
2021.
Low-Temperature Rheology and Thermoanalytical Investigation of Lubricating Oils: Comparison of Phase Transition, Viscosity, and Pour Point.
Lubricants,
Vol. 9,
Issue. 10,
p.
99.
Nouira, S.
Hassine, T.
Fitoussi, J.
Shirinbayan, M.
Gamaoun, F.
and
Tcharkhtchi, A.
2022.
Non-isothermal crystallization kinetics and its effect on the mechanical properties of homopolymer isotactic polypropylene.
Journal of Polymer Research,
Vol. 29,
Issue. 1,
Pezold, Daniel
Wimmer, Marco
Alfayez, Fayez
Bashir, Zahir
and
Döpper, Frank
2022.
Evaluation of Polyethylene Terephthalate Powder in High Speed Sintering.
Polymers,
Vol. 14,
Issue. 10,
p.
2095.
Thomsen, Thore Bach
Hunt, Cameron J.
and
Meyer, Anne S.
2022.
Standardized method for controlled modification of poly (ethylene terephthalate) (PET) crystallinity for assaying PET degrading enzymes.
MethodsX,
Vol. 9,
Issue. ,
p.
101815.
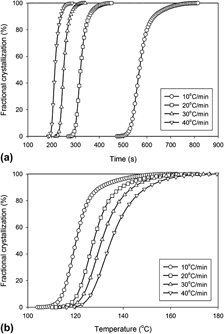
 increased exponentially with temperature; activation energies were estimated using Arrhenius plots for the two PET crystallization regimes.
increased exponentially with temperature; activation energies were estimated using Arrhenius plots for the two PET crystallization regimes.



